Sample Evolution Of Primates Research Paper. Browse other research paper examples and check the list of research paper topics for more inspiration. If you need a religion research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our research paper writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The Primates, one of 20 extant orders of placental mammals, are of special interest because they include the human species (Homo sapiens). It should be noted at once that tree shrews, included in the Primates for most of the last hundred years, are now generally thought to have only a distant connection (if at all) and are hence allocated to their own order Scandentia. A recent review (Rowe 1996) portrayed 238 living primate species (excluding tree shrews) and the number is now some 250 and rising, partly reflecting the discovery of entirely new species but also following subdivision of existing species because of new information, notably molecular evidence. This is a dramatic increase over the 150 species listed in a leading handbook in the 1960s (Napier and Napier 1967). The order Primates also includes almost 450 fossil species (also progressively increasing), documenting the evolution of this group.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
The evolutionary history of primates is a striking example of adaptive radiation, in which descendants from an original common ancestor with a particular set of characteristics diverged and adapted to occupy different geographical areas and ecological niches. Following Darwin’s Origin of Species, this evolutionary radiation was reconstructed initially almost entirely from morphological (anatomical) features, but the comparative framework has been expanded gradually to include physiological, behavioral, ecological, chromosomal, and molecular characters.
1. Living Primates
The logical starting point for reconstruction of primate evolution is the array of living species, partly because recognition of the group arose from the study of living representatives, and partly because many features (notably chromosomal and molecular characters) are not fossilized. Known fossil relatives of living primates can be included subsequently in the overall framework, providing the benefit of a geological timescale. Later inclusion of fossil species in a preliminary phylogenetic tree based on living forms also permits use of palaeontological evidence to test its validity.
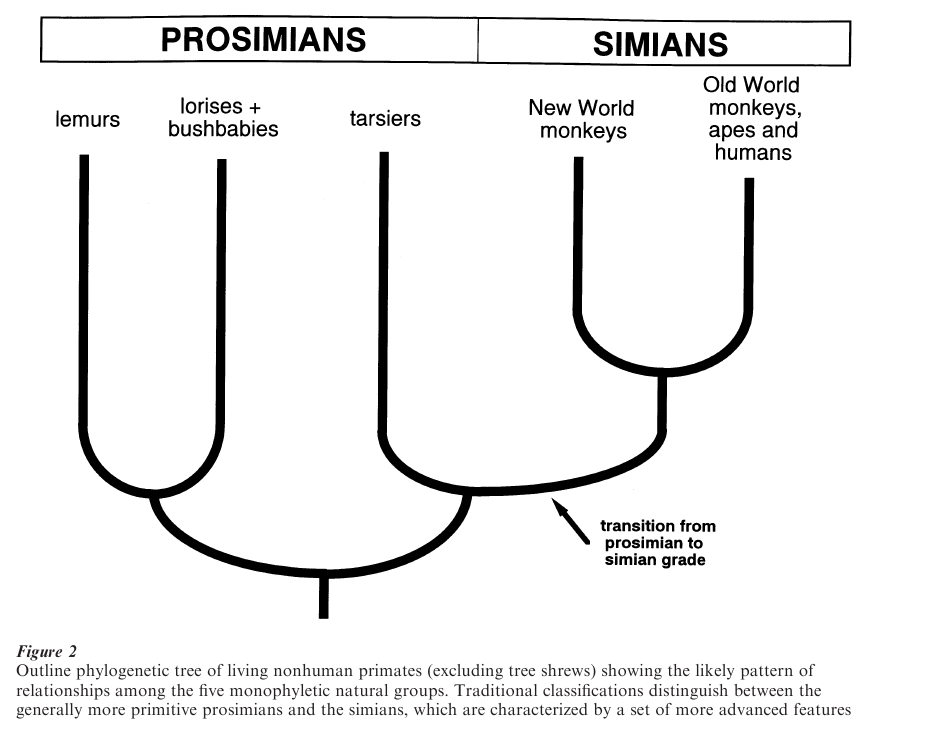
Living nonhuman primates can be divided into five ‘natural groups’, each with a restricted geographical range (Fig. 1). As geographical isolation plays a fundamental part in evolutionary divergence, these groups are inherently likely to reflect basic sub- divisions within the primate tree. Indeed, they are recognizable immediately in all widely accepted phylogenetic reconstructions and classifications. The first such group contains the Madagascar lemurs (infra- order Lemuriformes). These are the only primates present on that island, and document a substantial local radiation (Tattersall 1982). There are at least 35 living species and the modern array also includes at least 17 subfossil species that died out over the last few millennia, following the first human inhabitation of Madagascar. The second group contains the lorises and bushbabies (infraorder Lorisiformes). They are distributed widely in Africa, South Asia and South-east Asia, but the total number of species (about 20) is smaller than for lemurs, representing only a limited radiation. This applies even more drastically to the third group, containing the five closely related tarsier species of the South east Asian archipelago (infra- order Tarsiiformes). The fourth group contains the monkeys of South and Central America (infraorder Platyrrhini), which are the only New World primates. This is a relatively large group of some 80 species, divided into claw-bearing marmosets and tamarins (family Callitrichidae), and true monkeys (family Cebidae), again documenting a major local radiation (Kinzey 1997). The fifth group, containing the Old World monkeys and apes of Africa, South Asia and South east Asia (infraorder Catarrhini) is the largest, with approximately 105 species. In fact, humans also belong to this group, but have expanded far beyond this original geological range. Within this fifth group, which is most directly relevant to human evolution, there is a clear division between Old World monkeys (superfamily Cercopithecoidea) and apes and humans (superfamily Hominoidea), despite extensive overlap in geographical distribution.

The first three natural groups of primates (lemurs, lorises and bushbabies, tarsiers) have remained relatively primitive and are collectively labeled prosimians (literally ‘before the monkeys’). They are comparatively small (modal body weight approximately 500 g) and most species are nocturnal. Diurnal species (certain relatively large-bodied members of the families Lemuridae and Indridae) occur only among the lemurs. In fact, some lemurids (species of Eulemur and Hapalemur) show a highly unusual cathemeral pattern (Tattersall 1988), involving a mixture of nocturnal and diurnal activity. This is just one example of the great diversity of special adaptations found among the lemurs of Madagascar.
By contrast, members of the fourth and fifth natural groups of primates (monkeys, apes, and humans) share a distinctive set of advanced features in their dentitions, jaws, brain, and reproductive system, and can be labeled collectively simians. They are comparatively large (modal body weight approximately 5 kg), and virtually all species are diurnal. The only nocturnal simians are the New World owl monkeys (Aotus). The tenfold difference in modal body size between simians and prosimians, undoubtedly connected with the general distinction between nocturnal and diurnal habits, is just one indication of the pervasive importance of body size in primate biology. Availability of a compilation of body weights for almost all primate species, very largely derived from wild animals, therefore deserves special mention (Smith and Jungers 1997). Overall, living primates cover a 300-fold range of body sizes, extending from 40 g for the smallest of the mouse lemur species (Microcebus) to 120 kg for an adult male gorilla.
2. Definition Of Living Primates
It is often stated that living primates represent a graded series, ranging from relatively primitive to very advanced, and that it is therefore difficult to demarcate them from other orders of mammals (notably Insectivora). However, this view was influenced heavily by the inclusion of tree shrews in the order Primates. Reexamination excluding tree shrews revealed that there is in fact a substantial set of characteristics shared by all living primates, which separates them fairly clearly from other placental mammals, leading to the following definition (Martin 1986):
Primates are typically arboreal inhabitants of tropical and subtropical forest ecosystems. Their extremities are essentially adapted for prehension, rather than grappling of arboreal supports. A widely divergent hallux (big toe) provides the basis for a powerful grasping action of the foot in all genera except Homo, while the hand usually exhibits at least some prehensile capacity. The digits typically bear flat nails rather than bilaterally compressed claws; the hallux always bears a nail. The ventral surfaces of the extremities bear tactile pads with cutaneous ridges (dermatoglyphs) that reduce slippage on arboreal supports and provide for enhanced tactile sensitivity in association with dermal Meissner’s corpuscles. Locomotion is hindlimb-dominated, with the center of gravity of the body located closer to the hindlimbs, such that the typical walking gait follows a diagonal sequence (forefoot precedes hindfoot on each side). The foot is typically adapted for tarsi-fulcrimation, with at least some degree of relative elongation of the distal segment of the calcaneus, commonly resulting in reverse alternation of the tarsus (calcaneonavicular articulation).
The visual sense is greatly emphasized. The eyes are relatively large and the orbits possess (at least) a postorbital bar. Forward rotation of the eyes ensures a large degree of binocular overlap. Ipsilateral and contralateral retinofugal fibres are approximately balanced in numbers on each side of the brain and organised in such a way that the contralateral half of the visual field is represented. Enlargement and medial approximation of the orbits is typically associated with ethmoid exposure in the medial orbital wall (though there are several exceptions among the lemurs). The ventral floor of the well-developed auditory bulla is formed predominantly by the petrosal. The olfactory system is unspecialized in most nocturnal forms and reduced in diurnal forms. Partly because of the increased emphasis on vision, the brain is typically moderately enlarged, relative to body size, in comparison to other living mammals. The brain of living primates always possesses a true Sylvian sulcus (confluent with the rhinal sulcus) and a triradiate calcarine sulcus. Primates are unique among living mammals in that the brain constitutes a significantly larger proportion of fetal body weight at all stages of gestation.
Male primates are characterised by permanent precocial descent of the testes into a postpenial scrotum; female primates are characterised by the absence of a urogenital sinus (i.e. urinary and reproductive tracts entirely separate). In all primates, involvement of the yolk-sac in placentation is suppressed, at least during the latter half of gestation. Primates have long gestation periods, relative to maternal body size, and produce small litters of precocial neonates. Fetal growth and postnatal growth are characteristically slow in relation to maternal size. Sexual maturity is attained late and life-spans are correspondingly long relative to body size. Primates are, in short, adapted for slow reproductive turnover.
The dental formula exhibits a maximum of 2.1.3.3/2.1.3.3. The size of the premaxilla is very limited, in association with the reduced number of incisors, which are arranged moretransversely than longitudinally. The cheek teeth are typically relatively unspecialized, though cusps are generally low and rounded and the lower molars possess raised, enlarged talonids.
In fact, an additional distinctive feature should be added to this definition (Sussman 1999): all living primate species live in fairly elaborate social networks. Although nocturnal primate species have commonly been labeled ‘solitary,’ numerous field studies have shown that they have well established patterns of social interactions based on shared ranges, communal nesting, and regular encounters during the night. There are no immediately recognizable social groups of the kind found in all diurnal primates except the orang-utan, but there are nonetheless long-lasting associations between individuals. The pronounced social tendency now recognized for all primates is presumably linked to the high investment in individual offspring (including intensive parental care), and relatively long lifespans that characterize primates.
The distinctive set of characteristics shared by living primates reflects a suite of adaptations developed in their common ancestor. Taken in combination with various primitive features inferred for the ancestral condition, such as relatively small body size and nocturnal habits (Martin 1990), they yield a plausible reconstruction of the earliest primates. These were probably small-bodied, nocturnally active arboreal creatures that were particularly well adapted for exploitation of the fine branch niche, where they fed on a mixed diet of insects and fruit (the latter accounting for certain modifications of their molar teeth). Leaping and running in the fine terminal branches of trees and among lianas would account for their typical nail-bearing, grasping extremities, notably the prehensile foot, and for emphasis of the visual sense, with large, forward facing eyes and specialization of the brain for highly effective visual orientation. Development of prehensile extremities also involved a shift toward use of the hands rather than the snout in grasping food items, thus explaining shortening of the snout and the associated reduction in number of incisors. Adaptations for low reproductive turnover and high investment in individual offspring can be interpreted as responses to life under the relatively stable and predictable conditions of tropical forests combined with comparatively low predation levels among fine branches.
3. Phylogenetic Relationships Among Living Primates
In their broad outlines, reconstructions of the phylogenetic tree of primates based on morphological evidence have been confirmed by analyses based on chromosomal or molecular data, such that a general consensus is now emerging (Martin 1990, Fleagle 1999). Although certain relationships in the tree remain controversial, notably in cases where very early divergences are involved, such confirmation derived from largely independent analyses of new data provides valuable support for earlier phylogenetic reconstructions based exclusively on morphological evidence.
The substantial body of evidence now available indicates that the five natural groups of living primates (lemurs; lorises and bushbabies; tarsiers; New World monkeys; Old World monkeys, apes, and humans) are, in fact, phylogenetically distinct subunits within the tree (Fig. 2). In other words, each of the five natural groups is monophyletic (derived from a specific common ancestor within the tree). Although certain cranial features led some authors to suggest that the mouse and dwarf lemurs (family Cheirogaleidae) are more closely related to lorises and bushbabies than to other lemurs, chromosomal and molecular evidence indicates overwhelmingly that the lemurs are monophyletic (Yoder 1997). Furthermore, it has consistently emerged that the Old World monkeys, and the apes and humans, respectively, belong to two separate monophyletic groups at a lower level. The overall pattern of relationships between the major groups of primates has also become relatively clear. On the one hand, it has emerged consistently that the Lemuriformes and the Lorisiformes are sister groups (that is, their individual common ancestors were derived from an earlier common ancestor). Together, the lemurs, lorises, and bushbabies constitute a larger-scale monophyletic group known as strepsirrhines, because of their common retention of a rhinarium (a naked, moist area of skin surrounding the nostrils in the primitive mammalian condition). On the other hand, it has emerged equally consistently that the simian primates (monkeys, apes, and humans) together constitute a second larger-scale monophyletic group. There is also a considerable body of evidence indicating that tarsiers and simians are sister groups, together constituting a high-level monophyletic group known as haplorrhines, sharing complete suppression of the rhinarium (unique among mammals). Although some investigators have questioned an ancestral link between tarsiers and the simians, an overall review of evidence (Martin 1990) indicated that haplorrhines do, indeed, constitute a monophyletic group (Fig. 2).
4. The Primate Fossil Record
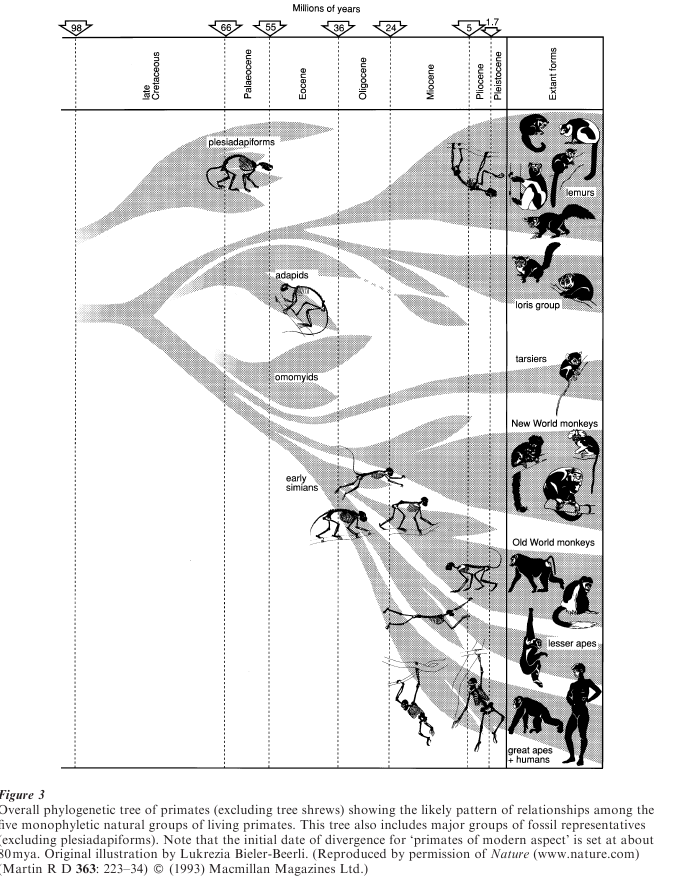
The known record of reliably identified fossil primates dates back to the beginning of the Eocene epoch, about 55 mya. An assemblage of earlier fossil forms mainly from the Palaeocene epoch (65–55 mya), collectively labeled ‘archaic primates’ (Plesiadapiformes), has traditionally been allocated to the order Primates. However, these early fossil forms (some of which survived into the Eocene) show few, if any, of the features in the definition given above and recent evidence indicates that their connection with primates is questionable. Even if Plesiadapiformes did have some early connection with primates, they surely diverged at such an early stage that they are of little relevance to the later adaptive radiation leading to modern primates and their direct fossil relatives (‘primates of modern aspect’).
The currently documented primate fossil record, excluding Plesiadapiformes, is divided fairly sharply into two segments separated by a gap of at least 6 million years in the middle of the Oligocene epoch (35–25 mya). Approximately equal numbers of fossil primate species (about 225) are known from each half of the record. Initially, primates of the Eocene epoch (55–35 mya) were documented only by early prosimians from Europe and North America. On both continents, they can be divided fairly clearly into two families, generally larger lemur-like Adapidae (e.g., Adapis in Europe, Notharctus in America), and generally smaller tarsier-like Omomyidae (e.g., Necrolemur in Europe, Shoshonius in America), and many authors have linked them to modern strepsirrhines and haplorrhines, respectively. Almost the entire skeleton is known for Notharctus; it is very lemur-like and certainly demonstrates that many defining features of modern primates were present at that stage.
This initial picture from America and Europe has been modified radically by subsequent fossil finds from North Africa, Asia and South east Asia. In North Africa, fossils from the latest Eocene and early Oligocene, notably from the Egyptian Fayum site, have documented the presence of a variety of early simians (e.g., Aegyptopithecus and Apidium) along with some potential relatives of modern strepsirrhines and tarsiers. More recently, Chinese middle Eocene fossil deposits have yielded evidence of early simians (Eosimias) and a putative direct ancestor of modern tarsiers (Tarsius eoceanus), along with adapids and omomyids. Furthermore, late Eocene sites in Myan-mar and Thailand have yielded several fossil simians (e.g., Amphipithecus and Siamopithecus). Thus, both prosimians and simians were present in the Eocene.
The situation is entirely different in the second half of the primate fossil record, extending from the late Oligocene to the present day. Direct fossil relatives of New World monkeys are known from South America, beginning in the latest Oligocene (Branisella) and extending into the Miocene (e.g., Homunculus). In the Old World (notably Africa), early Miocene deposits have yielded fossil relatives of bushbabies and lorises (e.g., Progalago), of tarsiers (albeit just one molar tooth from Thailand), and of monkeys and apes (e.g., Victoriapithecus and Proconsul ). Thus, four of the five modern natural groups of primates can be traced back to about 25 mya, and one (tarsiers) can be tentatively traced back to 40 mya. Nevertheless, there are also notable gaps in the known fossil record. The most striking gap is in Madagascar, where no single fossil from the major radiation of lemurs is known prior to the recently extinct subfossils. Similarly, in the New World, no single fossil form can be linked reliably to the marmosets and tamarins, a substantial proportion of the modern species.
Gaps in the fossil record influence directly the inferred date for the origin of primates. The standard practice has been to assume that primates diverged during the Palaeocene epoch, perhaps 60–65 mya, just a few million years prior to the first known fossil primates. However, direct inference from the known fossil record will lead to serious underestimation if there are substantial gaps in the fossil record, as is true of primates (Martin 1993). Several recent studies that calibrated molecular phylogenies for mammals using various dates for fossils of well documented groups outside the primates have all concluded that primates diverged from other mammals about 90 mya. Given some allowance for the elapse of time between this initial divergence and initiation of the adaptive radiation leading to modern primates, their common ancestor can be dated at about 80 mya (Fig. 3).
Bibliography:
- Fleagle J G 1999 Primate Adaptation and Evolution, 2nd edn. Academic Press, New York
- Kinzey W G (ed.) 1997 New World Primates: Ecology, Evolution, and Behavior. Aldine de Gruyter, New York
- Martin R D 1986 Primates: A definition. In: Wood B A, Martin L B, Andrews P (eds.) Major Topics in Primate and Human Evolution. Cambridge University Press, Cambridge, UK, pp. 1–31
- Martin R D 1990 Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton University Press, Princeton, NJ
- Martin R D 1993 Primate origins: Plugging the gaps. Nature, London 363: 223–34
- Napier J R, Napier P H 1967 A Handbook of Living Primates. Academic Press, London
- Rowe N 1996 The Pictorial Guide to the Living Primates. Pogonias Press, East Hampton, New York
- Smith R J, Jungers W L 1997 Body mass in comparative primatology. Journal of Human Evolution 32: 523–59
- Sussman R W 1999 Primate Ecology and Social Structure. Vol. 1: Lorises, Lemurs and Tarsiers. Pearson Custom Publishing, Needham Heights, MA
- Szalay F S, Delson E 1979 Evolutionary History of the Primates. Academic Press, New York
- Tattersall I 1982 The Primates of Madagascar. Columbia University Press, New York
- Tattersall I 1988 Cathemeral activity in primates: A definition. Folia Primatologica 49: 200–2
- Yoder A D 1997 Back to the future: A synthesis of strepsirrhine systematics. Evolutionary Anthropology 6: 11–22




