View sample cancer research paper on lung cancer. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Epidemiology of Lung Cancer
Lung cancer accounts for approximately 12.4% of all cancers diagnosed worldwide. There are estimated to be more than 1.35 million new cases each year, and this figure is steadily rising. From 1985 to 2005, the number of cases diagnosed annually increased by more than 50% globally. Nearly 1.2 million people die of the disease every year, accounting for 29% of total cancer-related deaths. In the United States and parts of Europe, lung cancer is responsible for as many deaths in men as the next three most common cancers (prostate, colorectal, and stomach) combined. Although incidence rates in women throughout the world are lower, lung is now the third commonest cancer site after breast and cervix. Furthermore, in certain regions lung cancer has overtaken breast cancer as the commonest cause of cancer-associated mortality among women.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Considerable geographical variation in the incidence and mortality rates of lung cancer exists, with the highest rates reported in industrialized nations. Whereas a peak in incidence appears to have been observed in Western and Northern Europe, rates continue to increase in Southern and Eastern Europe with overall age-adjusted incidence in males of more than 100/100 000 cases per year. Among black populations in several U.S. cities, the age-standardized incidence rate is above 100 cases per 100 000 of the population and lifetime risk exceeds 12%. In contrast, in parts of rural Africa and Asia, where exposure to lung carcinogens remains low, the age-standardized incidence rate is less than 5/100 000 annually. This figure is similar to that observed in parts of the United States and northern Europe at the start of the last century. Similarly, estimates from the International Agency for Research on Cancer (IARC) of age-standardized mortality rates for men and women show that there is considerable variation between regions (Figures 1 and 2). However, caution is needed in making direct comparisons of these data since differences exist in the methods of cancer registration in different geographical regions.
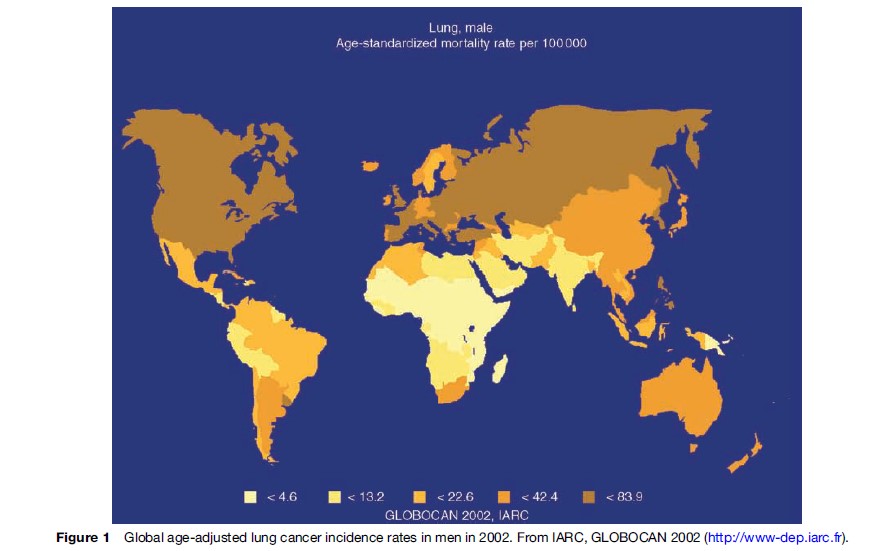
The International Agency for Research on Cancer (IARC) estimates the crude incidence rate of lung cancer for men in the developing parts of the world at 14/100 000 overall, although this likely represents a conservative estimate as rates of diagnosis and reporting are consistently lower than in industrialized countries. In 1980, it was estimated that the developing world accounted for approximately 30% of lung cancer cases worldwide. Recent data indicate that this figure is now closer to 50% of the total. Moreover, it is likely that lung cancer patterns will follow those observed in Europe and the United States during the latter twentieth century, with mortality rates predicted to reach almost epidemic proportions in the developing world during the next 50 years.
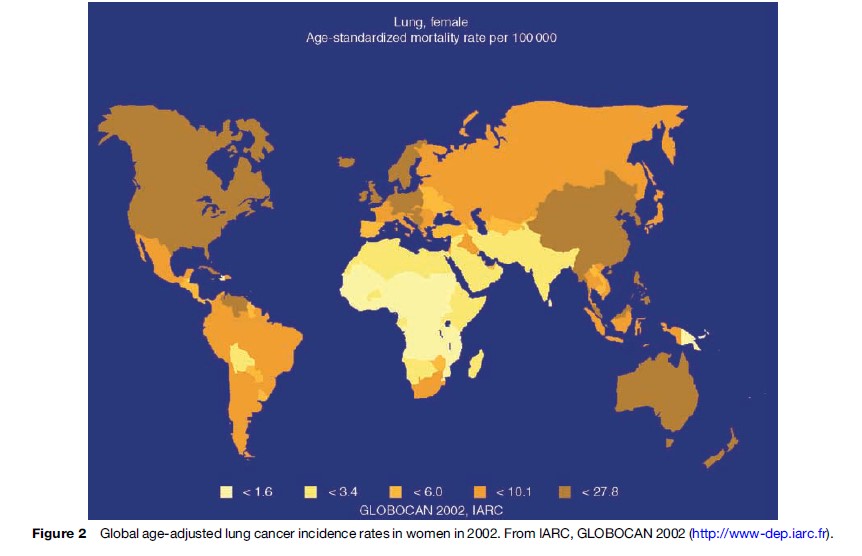
There is a striking correlation between social class and lung cancer. Those on lower levels of income and lesser attained education have disproportionately higher incidence rates, even after adjustment for smoking status. The reasons for these associations are incompletely understood, but likely reflect the complex interplay of important determinants of risk such as tobacco smoke exposure (both direct and environmental), dietary factors, and occupational carcinogen contact. There is also evidence that delays in diagnosis may also negatively contribute. In order to tackle this socioeconomic disparity, both basic scientific and epidemiological research endeavors are required. However, it is unlikely that significant gains will be made in this regard without effectively addressing the broader inequalities that exist between different social groups within society at large.
Risk Factors
Smoking
The link between tobacco smoke and lung cancer has long been suspected, but it was the landmark epidemiologic study by Doll and Hill published in 1950 that confirmed this association (Doll and Hill, 1950). Smoking is now recognized as the principal etiological factor in the overwhelming majority of cases; in developed countries, smoking is estimated to cause 87% of lung cancer deaths. Indeed, the indisputable role of smoking in causing lung cancer is among the most extensively studied causal relationships in biomolecular research. Each of the histopathological variants shows an association with smoking, with the strongest dose–response relationship seen for small cell carcinoma (see below).
The dramatic increase in the rates of lung cancer during the latter half of the last century closely mirrors patterns of tobacco consumption, with a lag time of approximately two decades. In 1998, it was estimated that more than 1.2 billion of the world’s population were smokers, approximately one-third of all persons aged 15 or older. Eight hundred million of these smokers are in developing countries. Worldwide, approximately 47% of all men and 12% of all women are current smokers, although there is considerable variation in this ratio between regions. In developed countries, 42% of men and 24% of women smoke, while in developing countries, it is estimated that 48% of men and 7% of women smoke, indicating that different nations are at different stages in the tobacco epidemic. Indeed, there has also been a notable decrease in smoking prevalence among men in many industrialized countries. This has had a significant impact on the numbers of lung cancers observed, which has shown a gradual decline during the last 20 years. In contrast, there has been a consistent rise in the numbers of new cases in women, reflecting an increase in smoking prevalence among women worldwide. Worryingly, even though the peak of lung cancer appears to have passed in males in the United States and parts of Europe in line with changing smoking trends, data indicate that no such peak has been reached in females (Devasa et al., 2005).
Environmental Tobacco Smoke
Environmental tobacco smoke (ETS), also referred to as passive or involuntary smoking, has been extensively investigated as a potential cause of lung cancer. The National Research Council in the United States estimates that 2–3% of all lung cancer (approximately 3000 cases per year) may be attributable to ETS. Much of epidemiologic research on ETS has examined the relative risk increase among nonsmoking women according to level of smoking by husbands. Overall there is an elevated risk of between 20 and 30% among nonsmoking wives of smoking husbands when compared with nonsmoking wives of nonsmoking husbands. Prolonged exposure to ETS in the workplace also confers increased risk and forms the basis for smoking-ban legislation in an increasing number of countries.
Factors Not Related To Smoking
Although smoking is implicated as causative in most cases, numerous other environmental and occupational exposures are known or suspected to contribute to the development of lung cancer. Data from investigations of occupational groups (often with prolonged exposure to physical or chemical agents at the workplace) estimate that 10–15% of lung cancers are linked to environmental factors. Furthermore, there is clear evidence that many carcinogens act synergistically with cigarette smoking to further augment risk. Even though the proportion of lung cancer unrelated to tobacco smoke is small, the absolute number of these cancers is significant and will remain a major burden in terms of overall cancer mortality, irrespective of the outcome of tobacco control endeavors.
One of the most important naturally occurring lung carcinogens is radon, a ubiquitous inert radioactive gas. Produced in the decay series of uranium, high levels of radon have long been linked to increased lung cancer risk. Underground miners exposed to elevated radon levels have particularly high rates of the disease. The U.S. Environmental Protection Agency and the Biological Effects of Radiation Committees estimate that approximately 15 000–20 000 cases of lung cancer deaths per year in the United States are due to radon exposure.
The link between asbestos and lung cancer was first made nearly 70 years ago. This association was strengthened by epidemiologic data from the 1950s showing that textile workers in Britain exposed to high levels of asbestos dust had a tenfold increased incidence. The risk conferred by asbestos exposure is increased in a multiplicative manner in smokers, due to a synergistic effect of several carcinogens found in cigarette smoke. Other proven or suspected occupational risk factors include nickel, chromium, beryllium, arsenic, and silica.
Both indoor and outdoor pollution have been implicated as etiologically relevant for lung cancer. Inhabitants of urbanized areas appear to be at increased risk, although there are considerable difficulties in obtaining reliable estimates of exposures. Inhalation of atmospheric contaminants may account for approximately 1–2% of lung cancers, although the presence of uncontrolled confounders in many studies may have influenced the strength of associations reported in the literature. Populations living in proximity to sources of high local pollution such as factories have also been extensively studied. In this regard, case–control studies showed that residents living in proximity to smelters of copper, zinc, or lead have increased risk of lung cancer, even after adjustment for smoking. Lastly, the widespread use of solid fuels such as unprocessed coal for cooking and domestic heating is believed to account for a significant proportion of cases among nonsmokers, particularly in developing countries.
Histopathological Subtypes
The World Health Organization (WHO) classification system of lung cancer recognizes four main cell types: Squamous cell carcinoma, adenocarcinoma, large cell (or undifferentiated) carcinoma, and small cell carcinoma. Each of these histopathological entities arises from transformed bronchial epithelium. As squamous cell, large cell and adenocarcinoma demonstrate overlapping clinical characteristics and anticipated treatment responses; they are grouped together under the umbrella term non-small cell lung cancer (NSCLC). Overall, NSCLC accounts for approximately 80% of all lung cancers, whereas small cell carcinoma (SCLC) accounts for approximately 15% of cases. Less common subtypes include carcinoid tumors and carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements.
The relative proportion of each of the four main histological types shows considerable variation between countries, although this is in part likely an effect of different methods and rates of tissue diagnosis. In North America, adenocarcinoma remains the most prevalent type seen, whereas squamous cell carcinoma is more common in Europe.
Differences between the relative proportions of pathological subtypes likely reflect not only changes in the prevalence of smoking among different populations but also modifications in cigarette design. In order to enhance the combustion of tobacco, modern cigarettes contain higher levels of nitrates. The result of more complete combustion is the generation of increased concentrations of the tobaccospecific nitrosamine, NNK, which is linked to increased risk of adenocarcinoma. Furthermore, smokers of filtered cigarettes with so-called low concentrations of tar (i.e., condensable residue of tobacco smoke) and/or nicotine tend to take larger average inhalational volumes in order to achieved desired levels of nicotine delivery. It has been hypothesized that this compensation in smoking pattern results in greater deposition of carcinogenic particulate matter to peripheral regions of the bronchial tree (Hoffmann et al., 1997), in turn increasing numbers of cancers that arise in peripheral airways and alveolar tissue.
Assessment Of The Lung Cancer Patient
Clinical Presentation
Most patients initially present symptomatically to their primary care physician or hospital emergency department, although approximately 5% of lung cancers are discovered incidentally. Symptoms may be caused by the primary tumor (e.g., hemoptysis or unexplained cough), regional invasion (e.g., chest wall pain), or distant metastatic spread (e.g., unexplained bone pain or new neurological symptoms).
Diagnostic Investigation
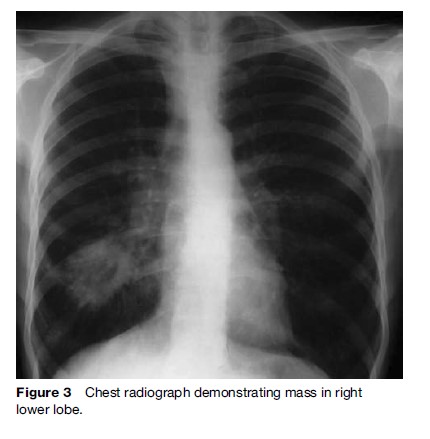
The purpose of investigation is twofold: To obtain tissue samples to confirm diagnosis and to determine the extent of disease dissemination (i.e., stage) in order to plan an appropriate therapeutic strategy. In general, thorough testing should be done in those being considered for aggressive treatment (e.g., lung resection or chemoradiotherapy) in order to exclude metastatic disease. The initial investigation of choice in cases of suspected lung cancer is the plain chest radiograph (Figure 3), which is invariably abnormal in those patients presenting with respiratory symptoms.
Tissue Diagnosis
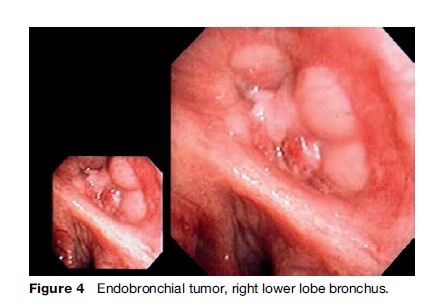
Tissue diagnosis is usually obtained via flexible bronchoscopy, during which essential endobronchial staging information is also obtained (Figure 4). Where the lesion is visible within the endobronchial tree, cytohistopathology specimens will almost always be diagnostic. For peripheral lesions beyond the visible portion of the airways, transbronchial samples taken under fluoroscopic X-ray guidance will be diagnostic in up to two-thirds of patients. Where samples from bronchoscopy are negative, percutaneous transthoracic needle aspiration biopsy performed under computed tomography (CT) guidance is a reliable diagnostic alternative.
Staging
In order to select the most appropriate therapeutic strategy, accurate staging information is mandatory. Bronchoscopy and CT scan of the thorax and upper abdomen are generally performed as a minimum, with further diagnostic testing undertaken as dictated by clinical assessment and/or initial imaging studies. In up to two-thirds of patients, clinical assessment alone will identify features that preclude radical therapy, either as a result of advanced disease or adverse patient factors such as impairment of lung function or comorbidities.
Spiral CT is established as the investigation of choice for characterization of the primary tumor, assessment of regional lymph node status, and detection of other intrathoracic pathology. A major drawback of CT is its relatively poor performance in accurately characterizing mediastinal lymph nodes. Overall published sensitivity rates range from 40% to 65%, with specificity rates of 45–90% reported. Positron emission tomography (PET) scanning is now also firmly established as a useful staging adjunct and may help identify the presence of occult metastases.
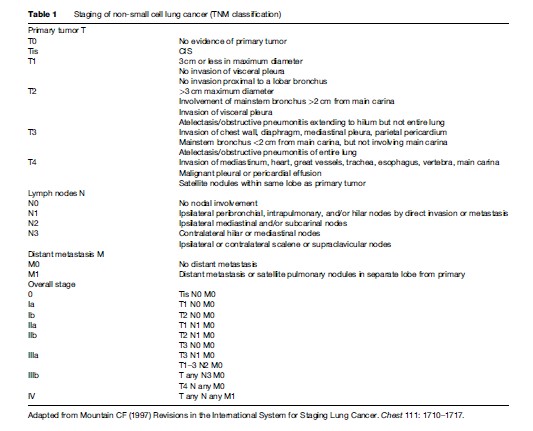
Patients with NSCLC are staged according to the international TNM (tumor, node, metastases) system (Table 1). This classification was published in 1997 by Mountain and is based on data from a U.S. database of 5319 patients assessed for surgery (Mountain, 1997). The TNM system is a powerful prognostic tool that correlates strongly with outcome and is used to determine the most appropriate therapeutic strategy. Application of the TNM system does not have the same prognostic relevance in SCLC, which is divided into limited (tumor is confined to a single hemithorax ± ipsilateral supraclavicular fossa) or extensive stage (tumor spread beyond limited stage).
Therapeutic Approach To Lung Cancer
The active management of the lung cancer patient should always begin with an assessment of that individual’s general medical condition and fitness for proposed therapy. Significant comorbidity, profound weight loss, and poor pulmonary function reserve may preclude aggressive treatment approaches.
Treatment Of Early-Stage NSCLC
The treatment of choice for patients with stage I and II NSCLC is surgical resection. Systematic mediastinal lymph node dissection at time of surgery improves accuracy of staging and may also confer a survival gain. In those with excessive surgical risk, stereotactic radiotherapy or radiofrequency ablation may be options for nodenegative tumors.
The optimal treatment for preoperatively confirmed stage IIIa disease and minimal N2 involvement remains controversial. Although regarded as inoperable by many, some series have shown a 20–30% 5-year survival with surgical resection in selected N2-positive patients.
There are now compelling data that adjuvant chemotherapy improves outcome in stage II and IIIa disease. A series of randomized studies have shown both overall and disease-free survival advantages using a platinum-based combination of agents in this setting. However, the benefit of chemotherapy for fully resected stage I patients remains unproven.
Treatment Of Advanced NSCLC
Those with locally advanced inoperable thoracic disease and adequate performance status should be offered combination chemotherapy/radiotherapy. Meta-analyses have demonstrated that combined modality regimens are associated with a small but statistically significant improvement in overall survival compared to thoracic radiotherapy alone. Appropriate candidates for this approach include stage IIIa patients with associated widespread N2 metastases and those with stage IIIb disease.
For individuals with stage IV disease (approximately 70% of total NSCLC burden), there is now ample evidence that treatment with palliative chemotherapy is superior to best supportive care. Combination treatment using a platinum-based doublet regimen should be considered as a first-line approach in those without significant weight loss or comorbidity. This strategy has been shown to offer improvements in quality of life and modestly increased median survival. Recently, novel targeted therapies such as erlotinib and bevacizumab have emerged as promising additions to the therapeutic armamentarium for advanced lung cancer.
Treatment Of SCLC
Patients with SCLC and adequate performance status should be offered combination chemotherapy; without treatment, median survival is less than 4 months. Response rates to chemotherapy in SCLC are excellent and a complete response (i.e., no evidence of residual disease) is achieved in more than two-thirds. However, relapse rates are high and long-term survival is unusual. Current recommendations are that those with limited-stage disease should also receive thoracic radiotherapy and prophylactic cranial irradiation, as this strategy reduces local recurrence and intracranial relapse, and confers a modest survival gain.
Survival
The EUROCARE, SEER, And NPCR Programs
In order to effectively plan the provision of public health services, accurate collection of data on survival rates for lung cancer patients is of critical importance. In the past, direct international comparisons were hampered by variations in classifications of cancers, as well as differences in methods and accuracy of data collection and statistical analysis. The establishment of large population-based cancer registries such as the EUROCARE program in Europe and the Surveillance, Epidemiology and End Results (SEER), and National Program of Cancer Registries (NPCR) projects in the United States has facilitated international comparisons of cancer management practices and of survival estimates. This in turn provides information that allows governmental agencies to plan and implement effective national cancer strategies and to improve delivery of health care for cancer patients. Moreover, these databases provide a robust method to gauge the success or failure of such strategies.
In 2003, Coleman and coinvestigators from the EUROCARE-3 project analyzed the outcomes of more than 250 000 cases diagnosed during the period 1990–1994 throughout Europe (Coleman et al., 2003). Results showed that there was no European country (for which data were available) in which 5-year survival for lung cancer was greater than 15%. Moreover, large differences in survival exist between and within European countries for lung cancer. In particular, there are significant differences in cancer survival rates between Eastern and Western European countries. Overall, survival rates are slightly higher in younger patients, though still less than 20% at 5 years. Outcomes for women were almost identical to their male counterparts (9.6% vs 9.7%, respectively). Published SEER Program data show an overall 5-year survival rate of 15%, which is marginally better than the European average reported in the EUROCARE-3 study.
Racial/Ethnic Disparity
A distinct racial/ethnic disparity exists in the burden of lung cancer, a problem that has been extensively studied in the United States. Findings of a report that combined surveillance data from both the SEER and NPCR projects indicate that substantial differences exist between Black Americans and non-Hispanic White Americans not only in incidence rates (109.0 vs. 86.8 per 100 000 per year), but also in survival. In 2007, Ries and Eisner published a summary of data from the U.S. National Cancer Institute database in which survival for more than 200 000 cases of lung cancer between 1988 through 2001 was analyzed. This revealed that the 5-year relative survival rate for White Americans was 16% and for Black Americans was 12%, a racial discrepancy that exists in both sexes and persists across all diagnostic stages.
The relative contributions from the various biological and/or societal factors that account for this ethnoracial survival gap have yet to be fully elucidated. Although differences in smoking patterns, tobacco metabolism, and susceptibility to carcinogenic effects of smoking have all been suggested as important considerations, data indicate that restricted access to diagnostic and therapeutic facilities due to socioeconomic factors (e.g., lack of medical insurance) also appears to contribute. Further study is necessary to establish the factors responsible for the significant difference in survival rates between the different ethic/racial groups.
Difficulties With Estimates Of Survival
Some of the practical difficulties in examining for changes in survival patterns over time are also highlighted by these data. From 1988 onward, patients with lung cancer and associated pleural effusion were classified as having distant disease rather than locoregional disease. As a result, there was an apparent increase in the proportion of patients with more advanced stage after 1988 according to the SEER database, making direct comparison of survival data difficult. Nevertheless, overall survival rates in the United States, Europe, and elsewhere have shown little change in the last 30 years.
Many experts criticize the use of improvements in 5-year survival rates as a valid measure of success of cancer prevention programs, since a number of biases (see below) can falsely indicate increased survival rates without an associated reduction in mortality. In the case of lung cancer, however, this point is somewhat moot, since the evidence generated from numerous large national cancer databases confirm consistently poor survival rates that have changed minimally over time in most countries. This is despite considerable advances in diagnostic techniques and refinements in therapeutic approaches during the past several decades.
Improving Delivery Of Lung Cancer Care
Approximately three-quarters of patients present with either regionally advanced or metastatic disease, and for the majority curative intent treatment is not possible. Given the enormous worldwide burden of disease, it is perhaps not surprising that the prevailing attitude to lung cancer, even among health-care professionals, is generally one of pessimism. However, the differences in international survival rates are attributable, in some measure at least, to factors that are amenable to intervention. It is likely that the variable survival rates reported throughout regions of Europe and the United States are in part explained by the considerable differences in the coordination and delivery of cancer services. In many parts of the world, such services are poorly organized and fragmented, such that the care offered to the individual patient may depend more on geographical location than clinical imperative.
In some countries, access to optimal care may be restricted due to socioeconomic considerations. For example, it is estimated that nearly 50 million people in the United States do not have medical insurance, and in 1988 Greenberg and coworkers showed there is evidence that lung cancer patients without private health insurance receive surgical (i.e., curative intent) treatment less often than those with private health insurance (Greenberg et al., 1988). This disparity in delivery of care impacts overall cancer survival rates. However, universally applied early diagnostic and therapeutic strategies would be expected to impact on the geographical and socioeconomic contributors to differences in survival rates. Indeed, there is ample evidence that improved organization of lung cancer services, with rapid access to multidisciplinary care, improves short and long-term outcome and enhances quality of life.
Future Estimates And Preventative Strategies
In the absence of dramatic future developments in early diagnosis or in therapeutic innovations, it is widely accepted that population patterns of smoking prevalence will remain the principal predictor of lung cancer mortality in the coming decades. In line with the decreases in smoking prevalence rates observed during the second half of the last century, so there has been a gradual reduction in incidence in many developed countries. In the United States, the numbers of smokers declined consistently from the 1960s until approximately 1990, at which point a plateau prevalence of approximately 25% was reached. Other developed nations have reported similar trends.
Given the lag time between population smoking patterns and associated lung cancer incidence, it is anticipated that a similar reduction in disease rates will be observed for the next 5–10 years, after which the prevalence will likely level off. The success of tobacco control programs will therefore be reflected in reduced burden of disease in most developed countries during the next decade. However, in order to continue the success of this downward trend, concerted efforts focused on sustained cessation and reduction of initiation are required.
There are only two ways to reduce cancer-associated mortality: By decreasing the number of incident cancers through primary prevention and by improving cure rates among those diagnosed with cancer through early detection and therapeutic advances. A plethora of well-designed, large-scale randomized studies have been conducted over the last 30 years or so, seeking to improve patient outcome by exploring various strategies based upon surgical resection, chemotherapeutic regimens, and radiotherapy approaches. Although these endeavors have helped clarify the roles of these different modalities in the management of lung cancer patients, most experts agree that a plateau of effect has probably been reached and it is likely that only modest further gains can be expected from the continued evaluation of treatment methods currently available. Yet lung cancer is almost entirely preventable, since the overwhelming majority of cases are due to a specific etiological agent, one that is, in theory at least, entirely avoidable. Thus, reducing the prevalence of cigarette smoking remains the most effective method of impacting on lung cancer burden worldwide.
Reducing The Prevalence Of Smoking
In order to impact on the prevalence of smoking, it is necessary both to prevent adolescent initiation of cigarette smoking and facilitate cessation among current smokers. There is clear evidence from cohort and case– control studies that individuals who stop smoking have a reduced risk of lung cancer compared to continuing smokers. Although the risk reduction is not immediate, the likelihood of developing lung cancer diminishes with the duration of cessation. There are also data to suggest that smokers who are unable or unwilling to completely quit nevertheless gain a reduction in risk by decreasing the number of cigarettes smoked. For example, among individuals who smoke 15 or more cigarettes per day, smoking reduction by 50% appears to significantly reduce the risk of lung cancer.
Through extensive and sustained tobacco control campaigns, most developed nations have achieved considerable success in reducing the proportion of citizens that smoke. In the United States, approximately half of all of those who have ever smoked are now former smokers. Some of this success has been attributed to the recent widespread introduction of bans on smoking in enclosed public places and workplaces in a number of different countries. The first of these was Ireland, and on March 29, 2004, the Irish Government implemented a complete ban on smoking in public places. Although the main reason cited for the introduction of such bans is to protect the health of workers, the encouragement of smoking cessation is accepted as an additional rationale. Unfortunately, the success of tobacco control programs in the developed world has led to increased marketing by tobacco companies in developing countries where lung cancer rates are rising dramatically.
Screening
Individuals who successfully quit smoking dramatically reduce their likelihood of lung cancer. Yet for many, a substantial risk remains. The reduction in smoking prevalence in many countries will have profound implications on the demographics of lung cancer in the future, as an increasing number of cases will occur in former smokers. In order to further reduce the risk for former (and current) smokers, there has been considerable interest for several decades in secondary preventative efforts such as screening. Most lung cancer patients present symptomatically with advanced disease and have a poor outcome. However, survival rates for those with surgically treated early-stage disease approaches 70% at 5 years. Thus, any screening strategy that detects more early stage disease might increase the proportion of potentially curable cases.
A number of conditions need to be met before a national screening policy can be implemented. The disease in question should represent a major public health problem with a high incidence and/or prevalence in the screened population. The screening tool employed must be safe, accessible, accurate, and cost-effective, and medical intervention at a preclinical stage of disease should lead to improved outcome. Most importantly, once an early detection program is established, both the disease-specific and overall mortality rates should be reduced in the screened population. Such measurements encompass the salutary effects conferred by earlier intervention as a result of screening and offsetting potential harm to those who choose to undergo unnecessary resection. This latter cohort comprises patients treated for spurious (i.e., falsepositive) test findings and those treated for disease that would remain nonlethal in the absence of intervention. It also includes those undergoing curative-intent treatments despite undetected occult metastatic disease.
Encouraging results from early nonrandomized chest X-ray screening studies led to a series of large randomized clinical trials conducted during the 1960s and 1970s. In these studies, volunteers were randomized to either a periodic chest radiograph or control arm after baseline examination. Investigators found an overall increased number of cancers with chest X-ray screening, but there was no reduction in death rates, even after an extended 20-year follow-up. Because of the relatively small enrollments, however, these studies may have overlooked a small but important benefit of annual chest radiography. As a result, and in an effort to definitively establish whether chest X-ray screening can impact on lung cancer mortality, the National Cancer Institute established the Prostate, Lung, Colorectal and Ovarian (PLCO) study, which enrolled nearly 155 000 participants. Although early results from the PLCO trial confirm that chest X-ray screening results in detection of a greater number of early-stage cancers, final results from analyses examining for an effect on mortality are awaited.
The advent of novel diagnostic techniques, together with the fact that methodological flaws may have introduced significant biases into earlier trials, has generated renewed interest in other early detection strategies. Of these, helical CT has shown greatest promise, and several studies evaluating the role of CT have been conducted in recent years. Overall, detection rates of early-stage tumors using CT screening are high, with the proportion of those with stage I disease approaching 90%. As a result, the proportion of patients undergoing potentially curative resection far exceeds rates of those of unscreened populations. Encouraging projected survival rates have also been reported with CT screening in the International Early Lung Cancer Action Program (I-ELCAP) study by Henschke et al. in 2006, although this was a nonrandomized trial that had no control arm (Henschke et al., 2006).
Unfortunately, screening strategies using CT have the major limitation of poor sensitivity. Depending on the study population, up to 85% of screened individuals will have irrelevant lesions detected with this strategy, most of which will be ultimately characterized as benign by serial scanning. However, a significant number of individuals will require invasive biopsy and occasionally thoracotomy where diagnostic uncertainty remains.
Data indicate that the mean volumetric doubling time for CT-detected cases of lung cancer is approximately 500 days, whereas that of non-screen-detected cases is only 100 days. This suggests that CT preferentially detects more indolent tumors, a phenomenon referred to as length-time bias. Positive results from studies may also be influenced by lead-time bias, in which an apparently improved survival time in a screen-detected cohort represents merely earlier detection of disease without impact on time of death. However, this latter bias may be avoided by designing properly conducted randomized studies with intervention and control arms that test lung cancer specific mortality as an endpoint.
Given that there remains a lack of conclusive data to show that screening using CT (or any other modality) reduces mortality, no country has yet introduced a population-based screening program for lung cancer. Ultimately, the question of whether a screening program using low dose CT is capable of reducing mortality should be answered by results from randomized, placebo-controlled trials currently underway. Most experts agree that such randomized trials are the only reliable method for obtaining true assessments of the benefits and potential harms of mass screening programs. In the United States, approximately 50 000 individuals have been recruited to the NCI-sponsored National Lung Screening Trial, with results expected in 2010. In addition, the NELSON trial is a European collaborative effort that also aims to evaluate the potential of CT screening through enrollment of 16 000 participants.
It is also important to note that, even if these studies show a positive effect that is attributable to screening, the cost-effectiveness of rolling out CT-based screening programs in different jurisdictions will require careful assessment. To date, published estimates using different computer-simulated models of the cost of CT screening have varied considerably, depending on the demographics of the populations screened (e.g., current or former smokers) and the outcome measure used. Whereas some studies have shown estimates in the region of $2500 per quality of life year (QALY) saved, other projections have indicated the cost to be closer to $2 000 000 per QALY saved. Ultimately, however, an accurate assessment of the cost-effectiveness of any screening technique can only be made if that technique is shown to save lives.
Bibliography:
- Coleman MP, Gatta G, Verdecchia A, et al. (2003) EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Annals of Oncology 14: v128–v149.
- Devasa SS, Bray F, Vizcaino AP, and Parkin DM (2005) International lung cancer trends by histologic type: Male: female differences diminishing and adenocarcinoma rates rising. International Journal of Cancer 117: 294–299.
- Doll R and Hill AB (1950) Smoking and carcinoma of the lung. British Medical Journal 2: 739–748.
- Greenberg ER, Chute CG, Stukel T, et al. (1988) Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. New England Journal of Medicine 318(10): 612–617.
- Henschke CI, Yankelevitz DF, Libby DM, et al. (2006) Survival of patients with stage I lung cancer detected on CT screening. New England Journal of Medicine 355: 1763–1771.
- Hoffmann D and Hoffmann I (1997) The changing cigarette, 1950–1995. Journal of Toxicology and Environmental Health 50: 307–364.
- Mountain CF (1997) Revisions in the International System for Staging Lung Cancer. Chest 111: 1710–1717.
- Ries LAG and Eisner MP (2007) Cancer of the Lung. In: Ries LAG, Young JL, Keel GE, et al. (eds.) SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. SEER Program, NIH Pub. No. 07–6215. Bethesda, MD: National Cancer Institute.
- Alberg AJ, Ford JG, and Samet JM (2003) Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines, 2nd edn. Chest 123(supplement 1): 21S–49S.
- Black C, de Verteuil R, Walker S, et al. (2007) Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax 62: 131–138.
- Clegg LX, Li FP, Hankey BF, et al. (2002) Cancer survival among US whites and minorities: A SEER (Surveillance, Epidemiology and End Results) Program population-based study. Archives in Internal Medicine 162: 1985–1993.
- Hackshaw AK, Law MR, and Wald NJ (1997) The accumulated evidence on lung cancer and environmental tobacco smoke. British Medical Journal 315: 980–988.
- Lubin JH, Boice JD, Edling C, et al. (1995) Lung cancer in radon- exposed miners and estimation of risk from indoor exposure. Journal of the National Cancer Institute 87: 817–827.
- Parkin DM, Whelan SL, Ferlay J, et al. (eds.) (1997) Cancer in Five Continents Vol. VII. International Agency for Research on Cancer (IARC) Scientific Publication no. 143. Lyon, France: IARC.
- Parkin DM, Bray FI, and Devesa SS (2001) Cancer burden in the year 2000: The global picture. European Journal of Cancer 37 (supplement 8): 4–66.
- Pass HI, Carbone DP, Johnson DH, Minna JD, and Turrisi AT (eds.) (2005) Lung Cancer: Principles and Practice, 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins.
- US Department of Health and Human Services (1989) Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services.
- The World Bank (1999) Curbing the Epidemic: Governments and the Economics of Tobacco Control. Washington, DC: The World Bank.
- https://www.who.int/health-topics/cancer.
- https://www.seer.cancer.gov.
- http://www.eurocare.it/.
- https://www.cancer.gov/types/lung/research/nlst.
- https://www-dep.iarc.fr/.




