This sample anthropology research paper on human brain features: 9100 words (approx. 30 pages) and a bibliography with 40 sources. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Whereas claims of human uniqueness used to revolve around the soul, they now revolve around the brain. Ever since Thomas Willis and his Oxford circle colleagues discovered in the late 1600s that the brain governs behavior, scientists have devoted considerable attention to this complex and inscrutable organ. Until recently, most approaches to the brain have been introspective and deductive. Philosophers and scientists traditionally have attempted to explain the brain’s workings by examining its current functioning. They study, in other words, modern minds. While this topdown approach has yielded many insights, the limitations are obvious. To understand the human brain, a historical or evolutionary approach is necessary. It is only by locating the brain in deep time and tracing its evolutionary development that we may hope to arrive at a more complete understanding of that which makes us uniquely human.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% OFF with 24START discount code
Background
Humans have large brains, in both absolute and relative terms. Presumably, it is this fact that prompted the normally restrained Charles Darwin to declare, in The Descent of Man (1871), that no one could possibly doubt the connection between large brains and higher mental powers. Whether Darwin was expressing a scientific truth or a cultural prejudice remains an open question. For reasons poorly understood and rarely questioned, humans are enamored of size—if something is big, it is usually deemed remarkable and important. In many instances, this habit of mind serves us well. Size often signals something important about function. In other instances, the privileging of size is misleading. This has been especially true of the human brain.
In the century after Darwin, it became commonly accepted that fish gave rise to amphibians, amphibians to reptiles, reptiles to mammals, and mammals to man. Implicit within this overly neat phylogenetic ordering was the idea that brain size increased with each phase change, and that each progression involved the addition of brain tissue. Indeed, it was this idea that inspired Paul MacLean’s “triune brain” model, which divides the brain into three parts (archipallium, limbic system, and neocortex) according to the sequence of their evolutionary appearance. Within the primate order, the story was much the same. As told by W. E. Le Gros Clark, primate evolution was largely a matter of progressive trends, one of which was expansion and elaboration of the brain. According to this traditional view, the most primitive primates (prosimians) had the smallest and simplest brains, more advanced primates (simians) had larger and more complex brains, and the most advanced primates (great apes) had even larger and more complex brains. Humans occupied the top rung of this primate scala naturae, and their brains were the biggest and most complex.
Against this backdrop, it should come as no surprise that those studying human evolution simply assumed, as Darwin himself seems to have done, that the transition from monkey to ape to human was largely a matter of growing bigger brains. Like most Victorians of his age, Darwin believed in progress, and his theory of natural selection reflected this belief. Firmly embedded within this progressivist paradigm, early anthropologists devoted themselves almost exclusively to the study of the brain, and more particularly crania. Paul Broca, who founded the Anthropological Society of Paris in 1859, contended that the new science of craniology was of such importance that anthropologists should focus exclusively on skulls. Speaking for many scientists of the day, Broca asserted that larger brains translated into greater intelligence. Given the prejudices of the time, this naturally meant that men had larger brains than women and Europeans had larger brains than Africans. In The Mismeasure of Man (1981), Stephen Jay Gould examined the evidence supporting these ideas and demonstrated that the data, if not simply made up or erroneous, supported none of these conclusions.
Obsessions over brain size have long vexed evolutionary thinking in general and anthropological studies in particular. In 1912, the discovery of fossils that came to be known as Piltdown Man seemed to confirm the idea that human evolution was largely a matter of growing bigger brains. Although there were good reasons to doubt the validity of the find, Piltdown’s large braincase fulfilled the a priori expectation that encephalization was the key to human evolution. Piltdown’s large brain cemented its status as a human ancestor. It took another 40 years before Piltdown was exposed as a hoax. During the interim, scientific acceptance of an actual fossil in the hominid lineage—Australopithecusafricanus, discovered by Raymond Dart in 1924—was long delayed because it did not possess a large enough brain.
Given this history, one might think, incorrectly, that anthropology has freed itself from its early focus on big brains. One of the ongoing debates in paleoanthropology revolves around the parameters of the genus Homo. Sir Arthur Keith, whose distinguished career ultimately was tarnished because he championed the big-brained Piltdown as a human ancestor, maintained that Homo should be defined by cranial capacity. For Keith and others this cerebral rubicon was 750 cubic centimeters. Any bipedal hominid below this threshold was not Homo and anything above it was Homo. Not everyone agreed with this definition, which became apparent in the 1960s when the Leakeys discovered a fossil cranium below the 750 cc threshold and named it Homo habilis. Nearly 50 years later, anthropologists are still debating the boundaries of our genus, with cranial capacities playing a prominent, albeit slightly reduced, role in those debates.
These controversies have not, in the end, done much to advance our understanding of the human brain. Part of the problem has been the narrow focus of these studies, which tend to orient themselves around fossil hominid skulls on the one hand and fully modern brains on the other. While this approach has merit, it is important to recognize that hominid brains have a much deeper evolutionary history. Any thorough understanding of the human brain requires some basic knowledge of primate brain evolution.
Primate Brain Evolution
The earliest fossil primates (adapids and omomyids) are approximately 55 million years old. Among a host of other diagnostic features for fossil primates, several significant ones involve cranial modifications that implicate the brain. In general terms, these derived characteristics include (1) brain enlargement, (2) enhanced vision, and (3) reduced olfaction. Because the insectivorous mammal that gave rise to primates remains unknown, it is difficult to determine whether brain enlargement is a valid descriptor of stem primates. The weight of evidence suggests that early primate brains were not enlarged and were comparable in size to primate sister taxa (Scandentia, Dermoptera, Chiroptera, Insectivora) at the base of the Archontan radiation. Although the earliest primate brains were not particularly encephalized, they were different. In basal primates, the extreme forward rotation of the eye sockets indicates an increased reliance on vision and decreased reliance on olfaction. Extreme orbital convergence suggests selection pressure for stereoscopic and binocular vision. A side effect of this convergence is that it constricts the space available for olfactory organs and their connections to the brain. Because early primates occupied arboreal habitats, the factors favoring enhanced vision may have included the need to locate branches for leaping-grasping locomotion, and the ability to prey on insects moving through the canopy.
Whatever the ultimate cause, there is no doubt that primate brains are visually specialized. Compared with those of other orders, a disproportionately large area of the primate brain is dedicated to visual processing. During the more recent course of primate evolution, the neocortex has expanded disproportionately. Because visual areas comprise approximately 50% of the primate neocortex, a great deal of this expansion is due to increased visual acuity. Primates have two distinct visual pathways in the brain: One (the magnocellular system) analyzes movement and form, while the other (the parvocellular system) processes detail and color. Visual area enhancement in early primates selectively altered the magnocellular system for detecting form and movement, whereas in later primates—including early anthropoids—the parvocellular system for discerning detail and color appears to have been selectively targeted. Because stem and early primates presumably were nocturnal and insectivorous, the enhanced development of the parvocellular system in later primates is often associated with an adaptive shift toward diurnality and frugivory. This shift, in turn, appears to have directly impacted the social behaviors for which primates are especially noted.
It is one thing for a small primate to hunt surreptitiously for insects at night and quite another to forage openly for fruit during the day. The former can be done in relative isolation and small groups, whereas the latter is best accomplished in the company of others and large groups. Social groups have several advantages, not the least of which is predator detection and defense. Complex social behavior requires considerable visual acuity—group members must be able to recognize one another, assess nonvocal behaviors, and respond accordingly. Group members must be able to process emotional and other states communicated through facial and gestural displays. It is not surprising, therefore, that there is a significant correlation between primate socialgroup size and neocortex size. There is a similar correlation between neocortex size and feeding ecology—frugivorous primates usually have larger neocortices than folivorous primates. This ecological correlation is typically explained in terms of mental mapping. Frugivory requires larger range size, with resources being patchy and temporal. The mental maps necessary to track these resources seem to require larger brains, larger groups, or both.
As is apparent, vision, sociality, and ecology interact in complex ways to alter the brains and behaviors of primates. This has been true throughout the course of primate evolution. Because none of these factors is uniform in space or time, primates have responded to these pressures differentially and variably. The resulting encephalization and reorganization has not, therefore, been uniform during the course of primate evolution. Encephalization and reorganization have occurred sporadically and independently within different lineages. Primate evolution has not simply been one long course of selection for bigger brains and increased intelligence.
Under the old classification scheme, the idea was that prosimians led to simians and simians to apes. With each supposed transition, there was a grade shift involving brains and behavior. Cladistic analyses have shown that this progressive phylogeny is no longer tenable. While it may be true that Strepsirrhines (lemurs, lorises, galagos) have smaller and less specialized brains than most Anthropoidea (a clade of primates including Platyrrhini and Catarrhini), this observation says nothing about supposed trends in primate brain evolution. Anthropoids did not evolve from Strepsirrhines; they are separate lineages, each with its own unique evolutionary history. Among anthropoids, Platyrrhines (New World monkeys) and Catarrhines (Old World monkeys and apes, including humans) display highly divergent patterns of encephalization and reorganization. Anthropoid brain evolution is, therefore, mosaic. There are no consistent directional trends, a fact made apparent by measures of encephalization for living anthropoids.
Brain Sizes and Encephalization Quotients
It has long been understood that absolute brain size, standing alone, says little about behavioral complexity. As body size increases, so does brain size. This is due in large part to the principle of proper mass, which holds that a certain amount of neural tissue is required to perform a particular function. Because larger animals have more intrinsic functions than smaller animals, they require larger brains to coordinate their autonomic, sensory, and motor activities. Whales, for example, have brains weighing thousands of grams. Their absolutely massive brains, however, do not make them more intelligent than primates, whose brains typically weigh hundreds of grams. Expressing brain weight as a percentage of body weight simply reverses the size problem. Using this ratio, small animals such as mice appear to be relatively more encephalized than whales and primates.
In an effort to correct these problems and identify some measure of brain-body size that correlates with behavioral complexity, Harry Jerison (1973) proposed the use of encephalization quotients, or EQ. The idea is straightforward—EQ is the ratio of an animal’s actual brain size to the brain size expected for an animal of its body size. On its face, EQ provides some measure of quantifiable objectivity. Despite this fact, EQs are neither straightforward measures of behavioral complexity nor definitive markers of intelligence. Embedded within EQ measurements are several assumptions, the most important of which is that there is a universally applicable, nonlinear scaling relationship between brains and body size. Researchers cannot, however, agree on the exponent that should be used to calculate EQ. Estimates vary widely from .20 to .75. Given this disagreement, the notion that an EQ value of 1.0 expresses a biological norm, or brain-size expectation given a certain body weight, is open to question. Consequently, EQ measurements and comparisons should be interpreted with caution. They simply serve as a useful first step in considering primate brain evolution and development. With these caveats in mind, Table 1 contains EQs for several species of extant anthropoid primates.
Table 1
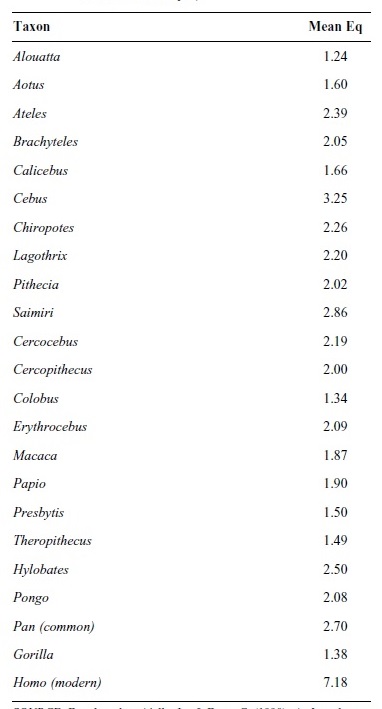
As is apparent, extant anthropoids are more encephalized than expected for mammals of similar body size. Despite this fact, EQ variation among anthropoids is great. Alouatta (howler monkeys) occupies the low end at 1.24 and Homo the high end at 7.18. Of particular interest is the fact that hominoid apes are not generally more encephalized than New or Old World monkeys. After humans, the primate with the highest EQ is not our closest relative the chimpanzee (Pan), but is instead the capuchin monkey (Cebus). Gorillas, for their part, are at the low end of EQs for anthropoids. Considered together, the EQ data should dispel the progressivist notion that apes are more evolutionarily advanced than monkeys. Judged by measures of EQ alone, this clearly is not the case.
When considering EQs, it is important to understand there is no such thing as a typical “primate brain.” Although all living primates share a common ancestor dating back to the Eocene, today there are nearly 300 extant primate species, each one of which has a unique evolutionary history. For each species, this history involves structural reorganization of the brain, along with changes in cell types, metabolic chemistry, vascular patterning, and neural connectivity. While some of these changes are the straightforward consequence of allometric enlargement, the majority cannot be so explained. The lack of any consistent pattern and the differences among species suggest that primate brains have undergone mosaic evolution, and there has been differential selection for particular kinds of behaviors. While primate brains may be similar in terms of gross morphology, this does not mean that chimpanzee brains are more sophisticated versions of macaque brains, or that human brains are simply scaled-up versions of chimpanzee brains. Each species has something unique about its brain. Given the many differences in primate ecologies and behaviors, this is not surprising.
Despite species-specific differences, there are some features of primate brains that appear to be unique to the order. Primates may be the only mammals that possess mirror neurons. These visuomotor neurons were first observed in macaques and are unique because they fire not only when an individual performs an intentional task (such as reaching for and grasping an object), but also when an individual observes another performing precisely the same task. Mirror neurons, in other words, appear to fire empathetically during the observance of purposeful acts performed by others. For this reason, mirror neurons have been linked to a range of primate specializations, including imitation, intentionality, agency, empathy, learning, and language. Taken together, these skills play a major role in social cognition. Significantly, mirror neurons also appear to play a major role in tool use. Although primates are not the only animals that have complex social systems and that use tools, the quality and complexity of primate behavior in these arenas differs from that of most other taxa. Another specialized neuron is found only among hominoid (i.e., great apes) species. Alone among primates, hominoids possess projection neurons known as spindle cells, found in the anterior cingulate cortex, an area associated with precision gripping and the regulation of cognitiveemotional processes. The remarkable fact that these cells exist only in our closest relatives (chimpanzees, gorillas, orangutans), in quantities that decrease as the phylogenetic distance from humans increases, suggests strong selection pressure for these specialized cells in our lineage.
Hominid Brain Evolution
Although progressive encephalization does not characterize primate brain evolution generally, it does characterize hominid brain evolution specifically. From Australopithecus to Homo, absolute brain size nearly tripled from an average of 450 cc to 1,250 cc. Some, but not all, of this expansion can be attributed to selection for increased body size. Correcting for body size and calculating EQ for hominids is not always easy, given that accurate estimates of body mass depend on postcranial remains. Because relatively few fossil crania are found with articulated or reliably associated postcranial remains, direct measures of body size are not often available. In their absence, researchers rely on various cranial proxies to estimate body mass. With these caveats in mind, Table 2 provides EQ values for selected hominids.
Table 2
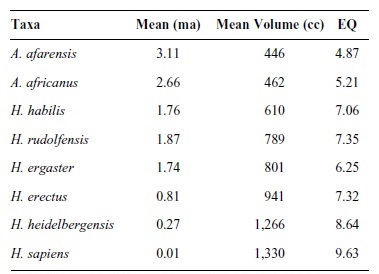
Because australopithecines had slightly larger brains and EQ values than living chimpanzees, modest encephalization is an appropriate marker (along with bipedalism) for the earliest hominids. As is evident from Table 2, absolute and relative brain sizes increased over time. Paleoanthropologists cannot, however, agree on the tempo and mode of hominid brain evolution. Some see steadily increasing cranial capacities and support a gradualist model. Others support a punctuated model and see an increase in cranial capacity with the appearance of early Homo, a long period of relative stasis, and another increase with the appearance of Homo sapiens. Regardless of which model is correct, two things should be kept in mind. First, our knowledge of within-species variation is lacking. Among modern humans, normal (i.e., nonpathological) brain sizes vary by as much as 1,000 cc (from 750 cc to 1,750 cc), without any apparent relationship to functioning or intelligence. Second, our sample sizes are small. For hominids, there are approximately 200 crania from which brain sizes can be reliably estimated. Of these, only 74 represent the human ancestral lineage if one assumes a phylogeny of A.afarensis,A.africanus,H.habilis,H.rudolfensis, H. ergaster, and H. erectus (Africa only) leading to Homo sapiens. Table 3 plots these 74 cranial capacity measurements without any adjustments for body weight.
Table 3
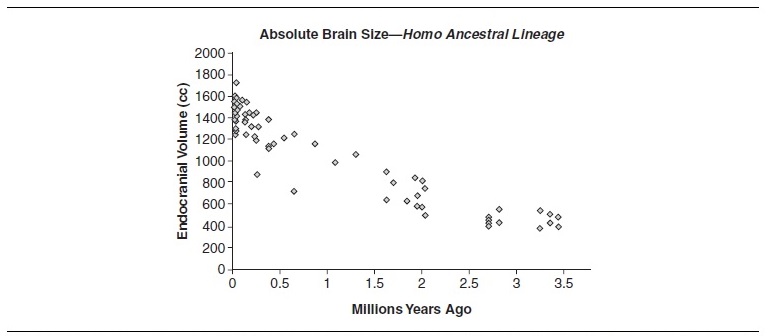
Regardless of how one chooses to characterize this scatter, the pattern of encephalization is clear, even after adjusting for body-size increases. Brain enlargement has several consequences, not the least of which is that it dramatically alters patterns of neural connectivity and developmental trajectory. Size, however, is not the only factor that can cause organizational change. The study of endocasts (molds of cranial interiors which reveal brain size and external morphology) shows that the hominid brain underwent significant reorganization over the last 3.5 million years. Although hominid brains are similar to most primate brains in terms of basic design (i.e., they are structurally homologous), the relative sizes of various structures have differentially enlarged or reduced over time. Among hominids, lateralization is an example of such change. Hemispheric lateralization is often associated with specialization of cerebral and motor function. Australopithecine brains show a hemispheric asymmetry that becomes more pronounced as one gets closer to Homo. A similar pattern characterizes the enlargement of Broca’s and Wernicke’s areas in hominid brains, both of which are associated with language. Another distinctive feature of hominid brains, vis-à-vis ape brains, is the relative reduction of the primary visual striate cortex and corresponding enlargement of the parietal lobe association cortex.
Considered together, these and other distinctive changes to hominid brains indicate two things. First, hominid brains in general and human brains in particular are not simply scaled-up versions of ape or chimpanzee brains. Second, differential enlargement and reduction of brain structures indicate a mosaic pattern to hominid brain evolution. This is an important point, given that natural selection does not see brain structures. Selection can only see behaviors that are mediated by brain structures. If those behaviors enhance fitness, then selection will favor those individuals whose brains are organized in a way that facilitates such behavior. Given the substantial changes in brain size and organization that occurred during the course of hominid evolution, identifying these behaviors— and associated selection pressures—has been the focus of much research.
Hominid Brain Evolution: Selection Hypotheses
It has long been fashionable to suppose that once hominid encephalization began, brain size steadily increased due to selection for “intelligence.” There are at least three problems with this idea. First, intelligence is inchoate and relative. As a scientific term, it lacks rigor and specificity. It cannot be directly measured. Many animals are intelligent, in the sense that their brains fully enable them to cope with the demands of their particular environments. Invariably, intelligence is assessed from a Homo-centric perspective blinding us to the considerable intelligence of other species. This issue aside, intelligence—as applied to humans—is a concept freighted with historical prejudice and modern bias. Although there are researchers who believe in a generalized intelligence (called g), these beliefs nearly always revolve around culturally specific and historically recent forms of cognition. Whatever these might be, they rarely are applicable to the kinds of cognitive demands made on hominids in Plio-Pleistocene environments. Second, neither absolute nor relative brain size is a reliable proxy for the behavioral complexity and cognitive plasticity associated with “intelligence.” Rats have small brains (~2 grams) yet have remarkable behavioral repertoires that enable them to adapt to all manner of environments. Among primates, capuchin monkeys have relatively much larger brains than gorillas, without major differences in assessments of intelligence. Finally, intelligence is simply too broad a concept to be useful. Rather than speaking in terms of general intelligence, we should discuss specific skills and abilities for which there may be evidence that is amenable to testing. So parsed, intelligence includes toolmaking, foraging, sociality, language, and culture. Before examining how these and other factors may have exerted selection pressure on hominid brains, it is important to remember that encephalization did not come first—bipedality was the prime mover in hominid evolution.
Although bipedality and the brain are often treated as separate and distinct aspects of hominid evolution, this approach ignores the major impact that bipedality had on the neural organization of hominids. All primates (except one) are quadrupeds of one kind or another. This means that for nearly 55 million years, primate brains have evolved in a manner that subserves the several different forms of quadrupedal locomotion. Standing upright requires substantial changes to this basic primate design. Bipedalism had direct and indirect effects on neural organization. The vestibular system, which plays a major role in balance and orientation, had to be reorganized, along with changes to neural pathways and associated motor regions. Several studies have shown that bipedalism is, over longer distances, an energetically more efficient form of locomotion than quadrupedalism. To maximize this efficiency and aid upright walking, the typically flared primate pelvis had to narrow. This reconstruction resulted in constricted birth canals, which in turn altered developmental patterns in hominids. Hominid infants had to have smaller brains (and softer bones) at birth in order to pass through a narrow birth canal. Having smaller brains at birth delays development. In humans, this delayed development is much longer than it is in other primates. Delayed postnatal maturation (secondary altriciality) has several effects, not the least of which is that it prolongs dependency and enables learning. The cumulative impact of this life-history alteration should not be underestimated. In all likelihood, bipedality accounts for the slightly changed brain-body size ratios first seen in australopithecines.
Bipedalism had another major impact on hominids: It freed the hands for tasks other than locomotion. The earliest hominids almost certainly lacked the fine motor control for hands that we associate with later hominids. Over time, however, hominids would have begun using their hands for novel tasks, including stone throwing, toolmaking, extractive foraging, and gestural communication. All of these require an ability to sequence grasping activities in a deliberate manner, and would have resulted in a significant reorganization of related motor control regions in the brain. Indeed, many researchers hypothesize that activities such as stone throwing and toolmaking laid the neural substrate for the later emergence of language, which also involves finely controlled motor sequencing.
Of course, bipedalism did not just happen. There had to be selection pressure for upright walking. Most researchers agree that upright walking began during a period of cooling and drying that resulted in the retreat of African forests and the appearance of patchy savannah-like environments. This ecological shift resulted in changed foraging patterns, at least for hominids who were no longer restricted to arboreal habitats. Bipedalism enabled hominids to range over larger territories, where foraging opportunities are much broader than they are in the canopy. Several changes in hominid-foraging patterns have been suggested, including opportunistic scavenging, cooperative hunting, tuber extraction, and shoreline harvesting. Whether early hominid foraging included all or only a few of these, each of these behaviors entail increased caloric intake, which is a major factor in any consideration of brain evolution.
In energetic terms, brains are notoriously expensive organs. Although the human brain constitutes a mere 2.3% of body mass, it consumes approximately 23% of the body’s daily energy intake. Given the high-metabolic costs of maintaining neural tissue, there are serious constraints on brain size. Many researchers argue that removal of these constraints was the essential first step in hominid encephalization. There are several variants of this argument, the most well-known of which is Leslie Aiello’s expensive tissue hypothesis (Aiello & Wheeler, 1995). Aiello notes that digestive organs are metabolically costly, and that in order to grow larger brains, hominids had to make a trade-off between digestive and neural tissue. The evidence for a reduction in hominid gut size and corresponding increase in brain size is compelling. As hominid brain size began increasing, the shape of the rib cage and thorax changed from the wide and flared hominoid pattern (indicative of a large gut designed to process lower quality foods) to the narrow and barrel-like human pattern (indicative of a smaller gut designed to process higher quality foods). These changes, in turn, are often associated with increased consumption of animal proteins, which could have occurred by hunting or, more likely, by way of scavenging and the secondary processing of carcasses and bones with stone tools.
Another group of researchers argue that this foraging and encephalization shift was associated with what they call the shore-based diet. Under this scenario, early hominids began exploiting the easy-to-harvest marine resources that are concentrated around the shorelines of lakes and rivers. These resources would have included crustaceans, mollusks, frogs, turtles, spawning fish, and birds’ eggs. In addition, a wider variety of edible and nutritious plants are available near water, and these presumably were included in the diet. A key feature of this argument is that shore-based diets include considerable amounts of fatty acids (docosahexaenoic acid and arachidonic acid) that are essential for encephalization in mammals. Importantly, these are limiting nutrients for brain development. However, these constraints were removed, and there can be little doubt that a higher quality diet was an important factor in hominid brain evolution. Recognizing this, Richard Wrangham recently has suggested that cooking—which results in much higher availability of nutrients from food—played a significant role in hominid evolution.
All constraint hypotheses have something in common: They revolve around changes in hominid behavior. As a general evolutionary rule, behavior remains constant unless something causes it to change. When environments remain stable over long periods of time, there is little reason for previously adaptive behaviors to change. However, when environments become variable and fluctuate rapidly, formerly adaptive behaviors may become maladaptive.
Organisms with relatively stereotyped and static behaviors that are unable to adapt thus become extinct. With this in mind, several researchers propose that behavioral plasticity in the face of environmental change was an important factor in hominid evolution. During the late Pliocene and throughout the Pleistocene, climactic change became more frequent and severe. Africa in particular experienced environmental perturbations that dramatically altered ecologies and landscapes. These changes would have exerted strong selection pressure for flexible and fluid responses, or an ability to behave in nonstereotypical ways. Because behavioral plasticity is often correlated with encephalization, habitat instability and hominid brain evolution are probably linked.
The Social Brain
In 1976, Nicholas Humphrey published a seminal article titled “The Social Function of Intellect.” Humphrey began with what appeared to be a paradox: Primates are among the most cerebral of animals, yet for the most part lead relatively undemanding lives. This thought occurred to Humphrey after spending a few months observing gorillas (and reading primate behavioral literature), all of which suggested that— compared with many other mammals—primate life (and in particular, foraging) was not especially difficult. Humphrey is not alone in this observation. Primatologists routinely confirm that field studies can be tedious, with long days spent watching primates leisurely foraging in trees or on the ground, alternated with long periods of rest and sleep. Given this fact, the question naturally arises: Why are primates so behaviorally sophisticated? For Humphrey, the answer was obvious. Primates are highly cerebral because they are intensely social.
Sociality is a complex evolutionary adaptation. One should not mistake mere aggregations of organisms with sophisticated social behavior. Life in a swarm, flock, or herd is in a limited sense social, but it does not involve the cognitive computations required of highly social mammals such as primates, cetaceans, and some carnivores. Complex social behavior usually involves tightly bonded groups or societies. Maintaining group cohesion while simultaneously tracking and navigating rank orders, member coalitions, shifting alliances, and individual relationships is no easy task, and is one that requires a fair degree of cerebral sophistication. Because relevant information must be constantly updated and stored over long periods of time, complex sociality places tremendous loads on memory and recall. It should come as no surprise, therefore, that all socially complex animals score rather high in various measures of encephalization and behavioral plasticity. Primates are especially notable in this regard.
With these observations in mind, Robin Dunbar (1998) proposed the social brain hypothesis to explain the fact that primates have unusually large brains, given their body size, compared with other vertebrates. Primates consistently have EQs higher than those of most other taxa. Dunbar began by noting, as many researchers have done, that neocortical areas of the brain are associated with reasoning and consciousness, and that the neocortex has expanded disproportionately during primate and hominid evolution. Operating on the assumption that primate group size is a rough proxy for social complexity, Dunbar measured primate neocortex size and compared it with primate group size. He found a significant correlation between these variables, and concluded that neocortex size acts as a constraint on primate group size.
Sociality does not, of course, always involve cooperation. It often involves group competition, which can create selection pressure for the ability to deceive. Pursuing this idea, Richard Byrne (2000) has found that all primates (except for Strepsirrhines) have at least some ability to deceive. Byrne’s Machiavellian intelligence hypothesis proposes that complex cognition in primates arises, in part, from the need to out-compete group members. Apes appear to possess greater deceptive abilities than monkeys, a fact which causes Byrne to argue that absolute brain size—rather than relative brain size alone— is an important factor in primate cognitive evolution. Byrne’s hypothesis has two important features that extend beyond the confines of Machiavellian intelligence. First, he notes that social competition has an inherent feedback effect. Because deception engenders counterdeception and the behavior of others is constantly shifting, there may have been spiraling selection pressure for advanced social cognition. This idea is similar to the “red queen” effect that drives predator-prey adaptations in a coevolutionary arms race. Second, advanced deception—such as that seen in apes—requires a theory of mind, or an ability to imagine others’ mental states. Significantly, mirror neurons and theory of mind have often been linked. Although the evidence for true theory of mind in other primates remains controversial, researchers agree that theory of mind is a key human attribute that played an important role in hominid evolution.
The Linguistic Brain
There can be little doubt that what separates humans from all other primates is language. Although cases such as Kanzi (bonobo), Washoe (common chimpanzee), and Koko (gorilla) show that intensively trained apes have impressive communicative and lexical skills, they do not possess language as we know it. More importantly, apes neither intuitively learn language (as do human children) nor spontaneously invent it (as did humans). Primate vocalizations and gestural routines are a long way from being languages or even protolanguages. Knowing this, the linguist Noam Chomsky long maintained that human language was so unique that it had no precedents in the animal kingdom. Chomsky’s early approach was antiDarwinian and essentially held that an innate language module somehow appeared, fully formed, in humans. A less saltational but explicitly Darwinian form of this idea has recently been advanced for the FOXP2 gene. Given the complex and supramodal nature of language, it seems highly unlikely that it simply appeared at some serendipitous moment in hominid evolution. For something like language to have evolved, there must have been more or less constant selection pressure toward it for hundreds of thousands, if not millions, of years. For this reason, many researchers have suggested that hominid encephalization and language are closely connected. It is apparent, however, that simply having a large brain cannot explain language. If it could, we might expect whales and elephants to have language.
Early researchers into brain function and anatomy identified two regions as having special significance for language: Broca’s area in the frontal cortex and Wernicke’s area in the temporal cortex. While these areas are undoubtedly important, patients with lesions to these regions do not usually experience complete language loss. Total aphasias or loss of language have been reported in patients who experience severe subcortical damage, a fact indicating that language functions are widely distributed in the brain. More recent imaging studies in humans confirm that language cuts across many brain regions and involves multiple connections, some of which appear to be of relatively recent origin. Increased connectivity is frequently cited as a differential aspect of the human brain associated with language. In the end, language defies localization, and there is no “language module” in the brain.
Two leading researchers on brain evolution and function, Merlin Donald (1991, 2001) and Terrence Deacon (1990, 1997a, 1997b), argue that the expansion and reorganization of the hominid brain over the last 2 million years was driven not by language per se, but by specific abilities that eventually culminated in language. In Donald’s view, early Homo possessed two critical abilities not seen in other primates: fine motor control and voluntary memory access. Together, these abilities allowed for mimesis, which is an ability to rehearse and refine body action in a representational manner. Mimetic skill could have operated without language, and would have greatly enhanced social cooperation and learning. In Deacon’s view, these changes amount to symbolic thought—an ability to model the world in abstract ways and communicate with others, even without fully developed language. Although Donald and Deacon differ on details, they agree that pre- and protolinguistic skills (such as gesture and prosody) underwrote the unique course of human brain evolution. In support of this view, Robin Dunbar (1998) observes that primate group cohesion depends to a large extent on grooming and hypothesizes that language evolved as a form of social grooming.
The Conscious Brain
In many respects, the human brain is most remarkable for its conscious properties. Precisely what consciousness is defies easy description or explanation. For humans, it is often associated with attention, focus, and awareness. Francis Crick likens consciousness to a searchlight that deals with current tasks and conditions. Purposive intentionality, goal states, future planning, and voluntary decision making are all aspects of consciousness. Given our Homo-centric view of the world, many assume that consciousness is a uniquely human attribute. This view is mistaken. While humans possess a type of consciousness that is different, there is no reason to think that other animals are not conscious. Consciousness, in other words, exists along a phylogenetic continuum.
Whether consciousness itself is a direct product of selection or is an emergent feature of neural evolution remains a mystery. We know, however, that mobile organisms face special challenges as they operate in multidimensional environments. Sensory inputs must be coordinated with motor outputs in a stable arena of action. For smaller, slower, and less complex organisms, this coordination does not even require a brain, let alone something akin to consciousness. For larger, faster, and more complex organisms, a brain—and some form of consciousness—appears to be necessary. If this is the case, then it is not unreasonable to suggest that reptiles are minimally conscious and that mammals are moderately conscious. Conscious organisms are aware of the immediate environment, and depending on feedback, are able to adjust behaviors. In this sense, consciousness is a form of error correction and action modulation, and its adaptive utility is obvious. The ability to react rapidly to constantly and rapidly changing environments is critical to survival.
Many researchers refer to primary consciousness, which is most often noted in mammals and birds, and higher-order consciousness, which is typically associated with humans (and may be minimally present in some apes, elephants, and cetaceans). Primary consciousness revolves around a remembered present and involves episodic memory. Its activation requires an external or environmental stimulus. Higher order consciousness entails introspection and involves both short- and long-term memory. It is selfcueing and does not require external or environmental activation (though this often occurs). Higher order consciousness also entails causation and subjectivity, which is an awareness of self associated with agency. For humans, this aspect of consciousness is self-evident and manifests as a stable identity. For other species, its presence may be indicated by self-recognition in mirror tests. Chimpanzees, elephants, and dolphins all appear to recognize themselves when presented with mirrors.
Given the central role that consciousness plays in our waking lives, it is not surprising that many researchers locate it in a central part of the brain: the thalamocortical system. The thalamus is medially situated to integrate sensory inputs and motor outputs. It appears to be a kind of switching center, with huge numbers of reciprocal relay cells engaged in recursive and parallel signaling. Gerald Edelman (2003) calls these relay signals re-entrant interactions that take place in the thalamocortical dynamic core. Significantly, brain wave activity in this core fluctuates in accordance with attention. Because the thalamus is centrally situated, it mediates between subcortical and neocortical processes. Its location, therefore, probably serves as an integrating area for the normally stable platform we call “consciousness.”
The Emotional Brain
Because language and consciousness play a central role in human experience, many philosophers and scientists have assumed that the mind is fundamentally rational. They have, in other words, privileged conscious cognition over other brain processes. Although Friedrich Nietzsche and Sigmund Freud vigorously challenged this assumption with their respective inquiries into “drives” and the “subconscious,” modern neuroscience typically eschews systematic inquiry into affective or emotional states. There is, however, one group of researchers who argue that much of our behavior is attributable to subconscious routines operating outside of language and consciousness. They are the evolutionary psychologists. In its most extreme form, evolutionary psychology holds that most of what humans do is driven by subconscious routines that evolved for specific and narrow purposes during the Plio-Pleistocene. Although there is little neurobiological evidence to support the idea that the brain is divided into modules, softer forms of evolutionary psychology focus on emotions and provide important insights into brain function and behavior.
As is true of consciousness, emotions exist along a phylogenetic continuum. At their most basic level, emotions are bioregulatory urges that govern approach/aversion and appetite/withdrawal behaviors. These urges are often parsed into arousal categories such as seeking, rage, fear, panic, play, lust, and care. Emotions allow animals to register environmental conditions, map body states, and maintain homeostatic balances. Many kinds of organisms— mammals prominently included—possess these abilities. Emotions enable reflexive responses to environmental stimuli and therefore play a major role in behavior. Internal-drive states related to food, sex, and safety are critical to survival and reproduction, the two essentials of evolutionary fitness. These drive states are largely regulated by emotions operating at subconscious levels. For animals possessing only primary consciousness—those locked into the present—emotions are highly adaptive and unproblematic. For humans possessing higher order and reflective consciousness, emotions—while still adaptive— are considerably more complex and are often problematic.
In humans, emotions register initially as drives that are then mediated by more complex cognition (i.e., language and memory). Feelings proper are the result of emotionalcognitive interactions. There is, in other words, an affective coloring to all conscious experience. This is, however, a two-way street—cognitive processes can trigger emotional responses. It should be apparent, therefore, that emotions play a major role in human decision making. What may appear to be purely rational thought processes are nearly always inflected by feelings that originate in the emotional brain. Under various circumstances, emotions and feelings can completely overwhelm executive level or rational cognition. Sickness and love provide but two examples, a fact well-known to all great novelists. Pure reason, as such, almost surely does not exist.
In the brain itself, emotions are usually identified with the subcortical limbic system, including the cingulate cortex, amygdala, and hypothalamus. In phylogenetic terms, these are relatively ancient structures that are closely connected with visceral functions. Their combined activity often triggers neuroendocrinal (hormonal) cascades, which can bathe the entire brain in chemicals affecting all aspects of feeling and behavior. The cellular and neuronal activity of the limbic system is regular and consistent. Jaak Panksepp (2003) suggests that these subcortical systems are akin to analog (regular stream) signals, whereas higher cortical systems are digital (intermittent pulse) signals. Operating within the context of the larger brain, these subcortical structures have major impacts on temperamental states, moods, and habits. They also play a role in memory formation, given that emotions often serve as tags for particular events. When similar emotions or conditions are experienced subsequently, the memory flood that sometimes results is a product of this valence tagging.
The Cultural Brain
Among scientists who study the brain, there is an unfortunate tendency to study it in isolation, as if it existed and operated in a box. The brain, of course, is encapsulated within a body, and the body exists in an environment. For humans, this environment is particularly rich: It is called culture. Although other animals possess transgenerational, social learning abilities that give rise to local traditions, the human brain takes these abilities to unprecedented heights. It is often said, with good reason, that the paramount human adaptation is culture.
Although human brains are specifically wired for various tasks at a subconscious level, higher order consciousness enables flexible learning across nearly all domains of thought and action. Human brains are, in a word, plastic. At birth (and compared with other primates), a human infant’s brain is grossly underdeveloped. To reach a similar stage of development at birth compared with chimpanzees, human infants would need to gestate another entire term, or until 18 months. This underdevelopment has several consequences, not the least of which is that it renders human infants utterly helpless and sets the stage for prolonged dependency. During early development, enormous amounts of energy are devoted to the brain. During the first few years of an infant’s life, the rapidly growing brain consumes 60% of daily metabolic expenditure, a figure that stabilizes at approximately 20% later in life. None of this growth occurs in isolation: Human infants are constantly attended to and surrounded by conspecifics. Though it may be hard to discern, infants almost immediately begin imbibing this highly social environment. As a consequence, their brains literally develop in a cultural matrix.
As Merlin Donald (1991, 2001) poignantly observes, there is no such thing as an isolated mind. We can no more conceive of a brain independent of culture than we can of a body independent of environment. Brains severed from culture are not normal. Tragic examples of this essential connection are seen in cases where children have been socially isolated and neglected during their developmental years. These abused children typically suffer permanent impairment of linguistic, social, and other skills that most take for granted. Clearly, the brain undergoes profound changes during these early years and cannot develop properly unless embedded in a cultural environment. This enmeshment is so tight and constant that we sometimes underestimate the degree to which our minds are bound by culture. Symbolic thought, considered by many to be the key attribute of the human brain, does not develop as a matter of course. Rather, cultural programming is required before symbolic thinking can occur. Symbols, in other words, originate outside the brain. Cultural learning allows us to decode and manipulate those symbols, but only after they are internalized. Because experience and learning physically alter the brain and its connections, it can be said that culture actually instantiates itself in the brain.
Though we tend to associate “culture” with recent Holocene achievements such as literacy and mathematics, hominid brains and culture have long been locked together in an evolutionary embrace. Unfortunately, our view of Plio-Pleistocene hominid culture is limited by the fossil record of mostly stone tools and bones. While the lithics associated with the Oldowan and Acheulean tool industries can tell us something about the brains and behaviors of hominids, that something is necessarily limited. The hominids who manufactured and transported Oldowan tools certainly understood causation and possessed foresight in ways that chimpanzees do not. The later hominids who manufactured more refined Acheulean forms had further developed this anticipatory cognition and understood symmetry. Other than these kinds of limited insights, however, we do not know precisely what kinds of cultural innovations were fueling hominid evolution. The most likely explanation is that social, technical, and communicative skills were all under selection pressure, with the result being a distinctive form of hominid culture. Whatever this culture may have been, it surely was more rich and complex than the picture we can paint from stones and bones alone.
Several researchers suggest that the hominid brain and culture formed an evolutionary feedback loop, having a ratchet effect on both. With each behavioral change and cultural modification, selection pressures would have favored those best able to adapt, and those best able to adapt would have possessed the kind of neural plasticity that often leads to further behavioral change and cultural modification. The notion of a hominid brain-culture spiral receives support from the evolutionary theory of niche construction, which posits that organisms can modify their environments in ways that alter subsequent selection pressures. The standard view in evolutionary theory is that selection pressures emanate from the environment to shape organisms who live, more or less passively, in the setting which presents itself to them. Selection, in this view, is a process with causation flowing only in a single direction. With the use of stone, bone, and fire, there came a point at which hominids began to actively alter their environments in ways that influenced subsequent selection. Humans, of course, radically alter environments to suit their needs, with consequences for subsequent behavior. Culture, in this view, widens evolutionary pathways so that causation can flow in two directions.
Conclusion and Future Directions
The human brain has deep evolutionary roots extending back in time to the first vertebrate brain, which appeared during the Cambrian some 500 million years ago. One lineage of vertebrates—the mammals—developed a relatively distinct brain that is roughly homologous across mammalian orders. Except for olfactory reduction and visual enhancement, early primate brains were not notably different from other mammalian brains. Primate brain evolution is marked primarily by emphasis on visual acuity, and only slightly by overall enlargement. Selection for visual acuity in primates most likely was associated with the shift to diurnality, changes in foraging, and complex social behavior.
The earliest hominid brains are not especially enlarged, but show signs of lateral and vascular reorganization that are of uncertain behavioral significance. Gross morphology aside, there can be little doubt that the foraging-related shift to bipedality worked significant changes to the early hominid brain. Bipedality forced changes to motor-control regions and had a major impact on the timing of brain growth and development. It was only after these changes had occurred that the hominid brain began to progressively enlarge. Issues of tempo and mode aside, hominid encephalization was most probably related to behavioral alterations involving sociality, technology, and communication. Considered together, these changes encompassed a distinctive hominid culture.
With the appearance of Homo sapiens some 200,000 years ago, the human brain had—in terms of overall size and external appearance—attained its modern configuration. There is no reason to think, however, that the human brain has stopped evolving since that time. Indeed, evidence from genetics suggests that there have been several mutations implicating the brain within the past 40,000 years. Some of these mutations involve size and speech, while other more recent ones involve auditory regions. These latter mutations may be especially relevant to the appearance of fully developed linguistic skills.
Progressivism in evolutionary studies and prejudice within anthropology hampered many early studies into brain evolution. An obsessive focus on size and “intelligence” has prevented many researchers from seeing that primate and hominid brain evolution has been an irregular, mosaic affair involving changes in structure, function, connection, cells, chemistry, growth, and development. Only over the last several decades have researchers begun to consider the brain in an evolutionary context relatively free from cultural myopia. This context has provided promising insights into pathologies ranging from schizophrenia to autism.
With regard to the human brain, it can rightfully be said that never has so much been known about so little. Brains, while small and unimpressive in appearance, are incredibly complex organs. The human brain has approximately 100 billion neurons and over 100 trillion synapses. Although the brain’s physical, cellular, and chemical composition is relatively well understood, its ability to give rise to mind is not. In almost every respect, brain research is in its infancy. Consequently, future research possibilities into brains and behavior are nearly limitless. Going forward, anthropologists who hope to contribute to our understanding of the human brain and mind will need to be able to cross disciplinary boundaries and work with researchers in genetics, neurology, biochemistry, biology, zoology, paleontology, psychology, ethology, and philosophy. If we hope to unravel the marvelous mystery that is the human brain, the combined insights from these fields and others will be required.
Bibliography:
- Aiello, L., & Wheeler, P. (1995). The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Current Anthropology, 36(2), 199–221.
- Allman, J., & Watson, K. (2002). Two phylogenetic specializations in the human brain. The Neuroscientist, 8(4), 335–346.
- Byrne, R. (2000). Evolution of primate cognition. Cognitive Science, 24(3), 543–570.
- Byrne, R., & Whiten,A. (Eds.). (1989). Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford, UK: Oxford University Press.
- Cunnane, S., & Crawford, M. (2003). Survival of the fattest: Fat babies were the key to the evolution of the large human brain. Comparative Biochemistry and Physiology, 136, 17–26.
- Damasio, A. (1998). Emotion in the perspective of an integrated nervous system. Brain Research Review, 26, 83–86.
- Damasio, A. (2003). Looking for Spinoza: Joy, sorrow, and the feeling brain. New York: Harcourt Brace.
- Deacon, T. (1990). Rethinking mammalian brain evolution. American Zoologist, 30(3), 629–705.
- Deacon, T. (1997a). The symbolic species: The coevolution of language and the brain. New York: W. W. Norton.
- Deacon, T. (1997b). What makes the human brain different? Annual Review of Anthropology, 26, 337–357.
- Donald, M. (1991). Origins of the modern mind: Three stages in the evolution of culture and cognition. Cambridge, MA: Harvard University Press.
- Donald, M. (2001). A mind so rare: The evolution of human consciousness. New York: W. W. Norton.
- Dunbar, R. (1998). The social brain hypothesis. Evolutionary Anthropology, 6(5), 178–190.
- Edelman, G. (2003). Naturalizing consciousness: A theoretical framework. Proceedings of the National Academy of Sciences, 100(9), 5520–5524.
- Finlay, B., Darlington, R., & Nicastro, N. (2001). Developmental structure in brain evolution. Behavior & Brain Sciences, 24, 263–308.
- Gould, S. J. (1981). The mismeasure of man. New York: W.W. Norton.
- Holloway, R. (1996). Evolution of the human brain. In A. Lock & C. Peters (Eds.), Handbook of human symbolic evolution (pp. 74–116). Oxford, UK: Oxford University Press.
- Holloway, R., Broadfield, D., & Yuan, M. (2004). The Human Fossil Record: Vol 3. Brain Endocasts: The Paleoneurological Evidence (J. Schwartz & I. Tattersall, Eds.). New York: Wiley-Liss.
- Humphrey, N. (1976). The social function of intellect. In P. B. G. Bateson (Ed.), Growing points in ethology (pp. 303–317). Cambridge, UK: Cambridge University Press.
- Iacoboni, M., Molnar-Szakacs, I., Gallese, V., Buccino, G., Mazziotta, J. C., & Rizzolatti, G. (2005). Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology, 3(3), 1–7.
- Jerison, H. J. (1973). Evolution of the brain and intelligence. New York: Academic Press.
- Kirk, E. C. (2006). Visual influences on primate encephalization. Journal of Human Evolution, 51, 76–90.
- Kirkaldie,M.,&Kitchener,P.(2007).Whenbrainsexpand:Mindand the evolution of cortex. Acta Neuropsychiatrica, 19, 139–148.
- Leonard, W., Snodgrass, J., & Robertson, M. (2007). Effects of brain evolution on human nutrition and metabolism. Annual Review of Nutrition, 27, 311–327.
- Lieberman, P. (1984). The biology and evolution of language. Cambridge, MA: Harvard University Press.
- MacLean, P. (1990). The triune brain in evolution: Role in paleocerebral functions. Berlin: Springer.
- Merker, B. (2005). The liabilities of mobility: A selection pressure for the transition to consciousness in animal evolution. Consciousness & Cognition, 14, 89–114.
- Mithen, S. (1996). The prehistory of the mind: The cognitive origins of art and science. London: Thames & Hudson.
- Nimchinsky, E. A., Gilissen, E., Allman, J. M., Perl, D. P., Erwin, J. M., & Hof, P. R. (1999). A neuronal morphologic type unique to humans and great apes. Proceedings of the National Academy of Sciences, 96, 5268–5273.
- Northcutt, R. G. (2002). Understanding vertebrate brain evolution. Integrative & Comparative Biology, 42, 743–756.
- Panksepp, J. (2003). At the interface of the affective, behavioral, and cognitive neurosciences: Decoding the emotional feelings of the brain. Brain and Cognition, 52, 4–14.
- Panksepp, J., & Panksepp, J. (2000). The seven sins of evolutionary psychology. Evolution and Cognition, 6(2), 108–131.
- Pinker, S. (1997). How the mind works. NewYork: W. W. Norton.
- Povinelli, D., & Preuss, T. (1995). Theory of mind: Evolutionary history of a cognitive specialization. Trends in Neurosciences, 18(9), 418–424.
- Radinsky, L. (1974). The fossil evidence of anthropoid brain evolution. American Journal of Physiology and Anthropology, 41, 15–28.
- Schoenemann, P. T. (2006). Evolution of the size and functional areas of the human brain. Annual Review of Anthropology, 35, 379–406.
- Seth, A., & Baars, B. (2005). Neural Darwinism and consciousness. Consciousness & Cognition, 14, 140–168.
- Striedter, G. (2006). Précis of principles of brain evolution. Behavior & Brain Sciences, 29, 1–36.
- Tartarelli, G., & Bisconti, M. (2006). Trajectories and constraints in brain evolution in primates and cetaceans. Human Evolution, 21, 275–287.
- Wynn, T. (2002). Archaeology and cognitive evolution. Behavior & Brain Sciences, 25, 389–438.




